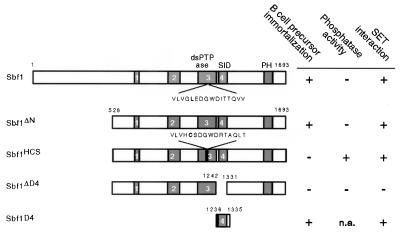
Figure 6.
Schematic illustrations of Sbf1 proteins with a summary of their growth-altering and biochemical properties. ░⃞, Protein domains conserved among myotubularin family members. Domain 3 displays similarity with the catalytic domains of tyrosine and dual-specificity phosphatases (dsPTPase); domain 4 (SID) mediates interactions with SET domain proteins. Sbf1 lacks several catalytically essential residues in domain 3 that were restored in construct Sbf1HCS. The growth-altering properties of Sbf1 constructs are based on their ability (+) or inability (−) to induce growth of nonadherent bone marrow cells exceeding 105 cells per ml after 16 days of culture under Whitlock-Witte conditions. The phosphatase and SET domain interaction properties of the various Sbf1 proteins have been reported elsewhere (14). n.a., not applicable.