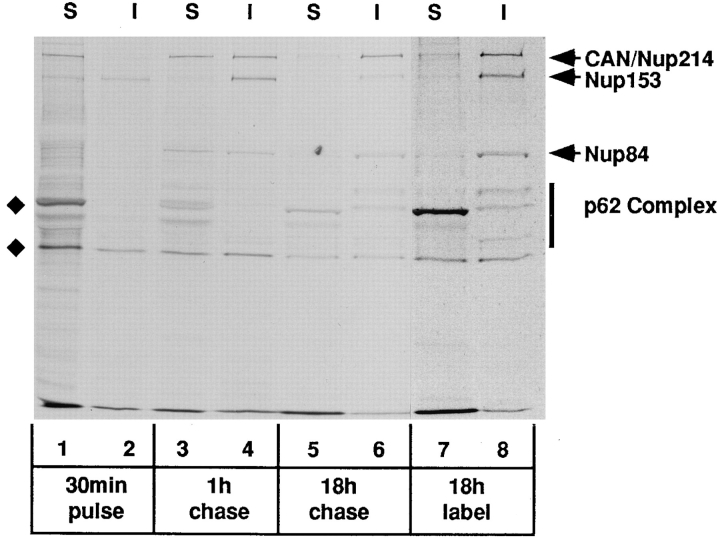
Figure 5.
Pulse–chase analysis of NPC proteins in BHK cells labeled with 35S-Trans label. The cells were either labeled continuously for 18 h (lanes 7 and 8) or pulse labeled for 30 min (lanes 1–6) followed by a “chase” of 1 h (lanes 3 and 4) or 18 h (lanes 5 and 6) in medium containing excess unlabeled methionine/cysteine. At the appropriate time, the cells were lysed in a low salt buffer containing Triton X-100. The distributions of CAN/Nup214 and Nup84 between soluble and insoluble fractions were then determined by immunoprecipitation analysis using the QE5 monoclonal antibody (which recognizes CAN/Nup214, Nup153, and p62). Total or bulk CAN/Nup214 (and associated Nup84) observed in the samples derived from the continuously labeled cells (lanes 7 and 8) is almost completely (80–90%) resistant to solubilization. In contrast, following the 30-min labeling period, 90% of newly synthesized CAN/Nup214 is found in the soluble fraction (lanes 1 and 2) and, furthermore, coprecipitates with Nup84. Both of these proteins are subsequently “chased” into the insoluble fraction with a half-time of about 1 h (lanes 3 and 4). These findings are consistent with the notion that the two proteins associate before stable integration into the NPC. While derived from the same experiment and run on the same gel, exposure times for lanes 1–6 versus lanes 7 and 8 have been adjusted (11 d and 8 h, respectively) to yield comparable intensities. The two bands indicated by the diamonds, the upper of which migrates just ahead of the p62-associated protein, p58, are both observed with nonimmune Sepharose beads.