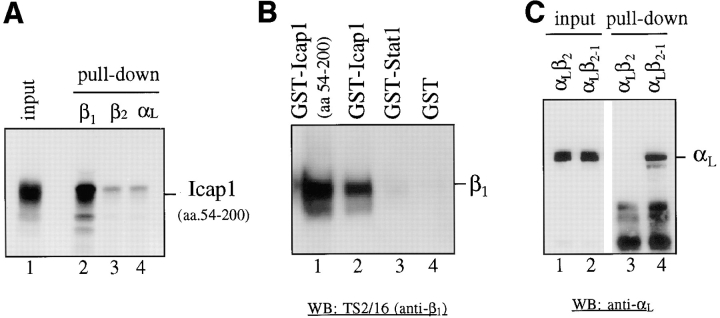
Figure 2.
In vitro and in vivo interaction between ICAP-1α and β1 integrins. (A) Interaction between GST-β1 and ICAP-1α in vitro. ICAP-1α (aa 54–200) was synthesized in vitro using reticulocyte lysate (lane 1) and incubated with 2 μg of bacterially expressed GST fusion proteins containing the cytoplasmic domains of integrin β1 (lane 2), β2 (lane 3), and αL (lane 4). (B) Interaction between GST-ICAP1α and β1 integrins in vivo. ICAP-1α (aa 54– 200) (lane 1) and a full-length ICAP1α (lane 2) were expressed as GST fusion protein in 293T cells using eukaryotic expression vector pEBG. For controls, GST-Stat1 (lane 3) and GST (lane 4) were used. Coprecipitation of the endogenous β1 integrins with the GST fusion proteins were determined on a Western blot using TS2/16 (anti–human β1 integrin antibody). (C) Restricted binding specificity of ICAP1α. GST-ICAP1α was expressed in 293T cells along with expression constructs for αLβ2 or αLβ2-1 (chimeric β2 subunit that has the COOH-terminal 20 aa replaced with the COOH-terminal 21 aa of β1 integrin). Coprecipitation of transfected integrins with the GST-ICAP1α was determined on a Western blot using anti-αL antibody. Lanes 1 and 2, total lysates of cells expressing GST-ICAP1α and αLβ2 (lane 1) and αLβ2-1 (lane 2). Lanes 3 and 4, the same samples as in lanes 1 and 2, respectively, after GST “pull-down.”