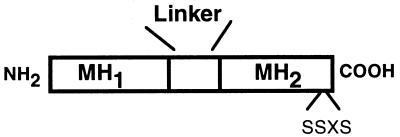
Figure 1.
Schematic of Smad protein structure. A general schematic of the structure of a human Smad protein is shown. Smad proteins consist of two domains (based on sequence conservation among identified Smad proteins) termed MH1 and MH2 (for MAD homology domain) located in their amino- and carboxyl-terminal halves, respectively. These regions are separated by a more variable, proline-rich domain known as the linker region. Certain signaling or receptor-associated Smads, such as Smad1 and Smad2, have a series of serine residues (termed the SSXS motif) present in their carboxyl termini, which are the sites of type 1 receptor-mediated phosphorylation. Human Smad4 and Smad7 do not possess the SSXS sequence.