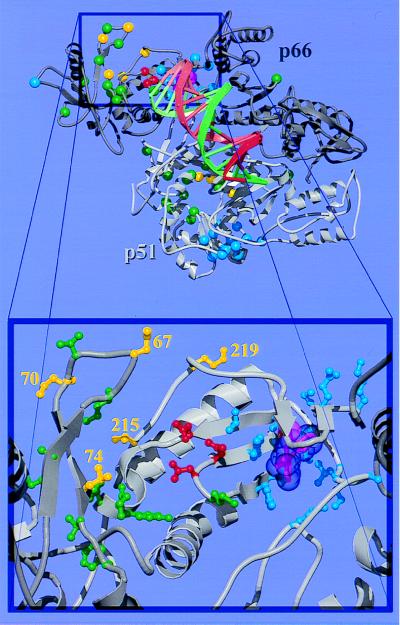
Figure 1.
Overall structure and drug resistance mutation sites of the RT heterodimer. (Top) The p66 subunit is drawn in dark gray and p51 in light gray. NI resistance mutation sites (26) are shown as green spheres, with RTMC and L74V sites highlighted in yellow. In the p51 subunit, residues 215 and 219 are disordered; their positions are not shown. NNI resistance mutation sites (27) are shown as blue spheres. The three polymerase active site aspartate residues and the bound NNI are shown in red and magenta, respectively. Double-stranded DNA (shown as a spiral ladder with the template strand in green and the primer in red) was modeled into our RT-nevirapine structure (6) from the Cα and phosphate coordinates of the RT-DNA-Fab complex (5) by superimposing the p66 palm domain of the two structures. (Bottom) A close-up view of the polymerase active site and the drug resistance mutation sites in the p66 subunit. The coloring scheme is the same as in the top panel; however, the side chains for mutated residues are shown in ball-and-stick representation and the van der Waals surface for the bound NNI (nevirapine) is shown semitransparent.