Abstract
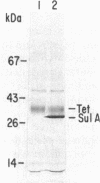
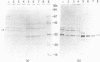
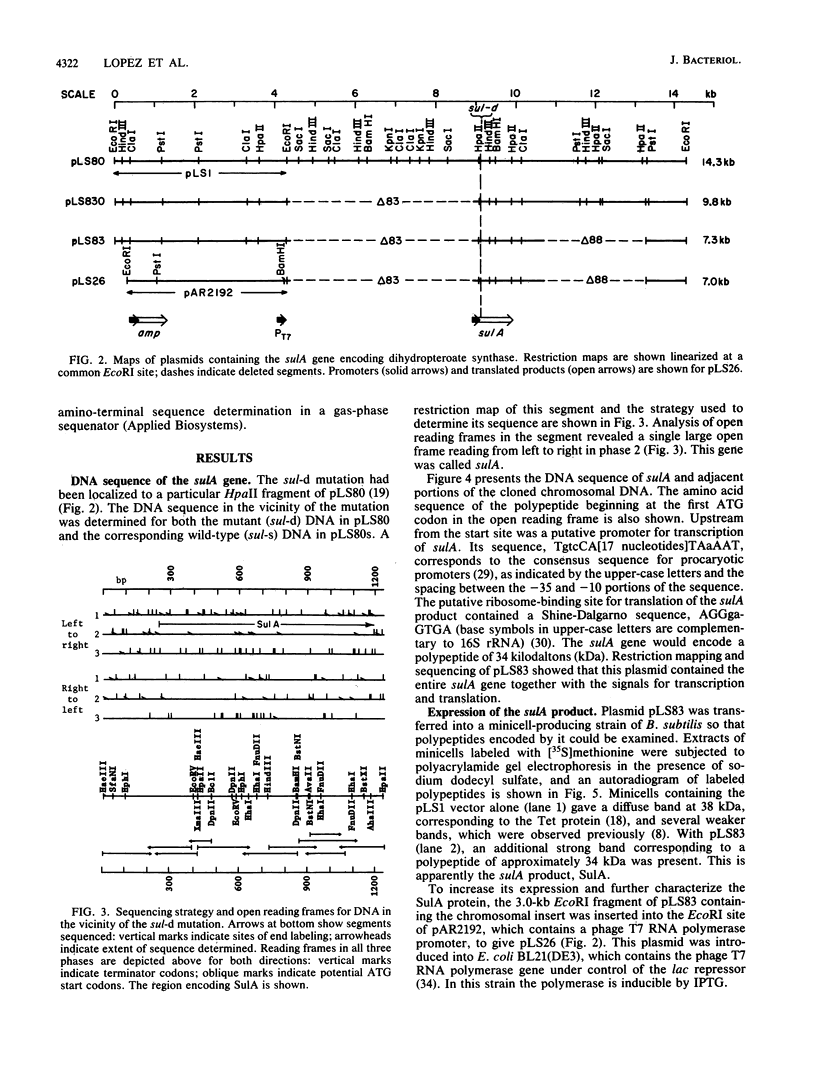
A chromosomal gene of Streptococcus pneumoniae carrying a spontaneous mutation to sulfonamide resistance was identified. Comparison of its DNA sequence with the wild-type sequence showed that the mutation, sul-d, consisted of an insert of 6 base pairs, a repeat of an adjacent 6-base-pair segment. The gene encoded a 34-kilodalton polypeptide, SulA, which as a dimer or trimer constituted the enzyme dihydropteroate synthase. This was shown by enzyme activity measurements, expression in minicells of Bacillus subtilis, and the amino-terminal sequence of the polypeptide product. Subcloning of the gene in an Escherichia coli expression vector allowed purification of the enzyme to 80% homogeneity in a single step and at high yield. Although a deleted plasmid, pLS83, produced the mutant dihydropteroate synthase, it did not confer sulfonamide resistance in vivo. It is suggested that the SulA polypeptide is also a component of an enzyme that acts in another step of folate biosynthesis and that this step is inhibited in vivo by either free or conjugated sulfonamides.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angier R. B., Boothe J. H., Hutchings B. L., Mowat J. H., Semb J., Stokstad E. L., Subbarow Y., Waller C. W., Cosulich D. B., Fahrenbach M. J., Hultquist M. E., Kuh E., Northey E. H., Seeger D. R., Sickels J. P., Smith J. M., Jr The Structure and Synthesis of the Liver L. casei Factor. Science. 1946 May 31;103(2683):667–669. doi: 10.1126/science.103.2683.667. [DOI] [PubMed] [Google Scholar]
- BROWN G. M. The biosynthesis of folic acid. II. Inhibition by sulfonamides. J Biol Chem. 1962 Feb;237:536–540. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Claverys J. P., Lacks S. A. Heteroduplex deoxyribonucleic acid base mismatch repair in bacteria. Microbiol Rev. 1986 Jun;50(2):133–165. doi: 10.1128/mr.50.2.133-165.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claverys J. P., Méjean V., Gasc A. M., Sicard A. M. Mismatch repair in Streptococcus pneumoniae: relationship between base mismatches and transformation efficiencies. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5956–5960. doi: 10.1073/pnas.80.19.5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Espinosa M., Lopez P., Perez-Ureña M. T., Lacks S. A. Interspecific plasmid transfer between Streptococcus pneumoniae and Bacillus subtilis. Mol Gen Genet. 1982;188(2):195–201. doi: 10.1007/BF00332675. [DOI] [PubMed] [Google Scholar]
- Espinosa M., López P., Lacks S. A. Transfer and expression of recombinant plasmids carrying pneumococcal mal genes in Bacillus subtilis. Gene. 1984 Jun;28(3):301–310. doi: 10.1016/0378-1119(84)90147-1. [DOI] [PubMed] [Google Scholar]
- FUTTERMAN S. Enzymatic reduction of folic acid and dihydrofolic acid to tetrahydrofolic acid. J Biol Chem. 1957 Oct;228(2):1031–1038. [PubMed] [Google Scholar]
- GRIFFIN M. J., BROWN G. M. THE BIOSYNTHESIS OF FOLIC ACID. III. ENZYMATIC FORMATION OF DIHYDROFOLIC ACID FROM DIHYDROPTEROIC ACID AND OF TETRAHYDROPTEROYLPOLYGLUTAMIC ACID COMPOUNDS FROM TETRAHYDROFOLIC ACID. J Biol Chem. 1964 Jan;239:310–316. [PubMed] [Google Scholar]
- HOTCHKISS R. D., EVANS A. H. Analysis of the complex sulfonamide resistance locus of pneumococcus. Cold Spring Harb Symp Quant Biol. 1958;23:85–97. doi: 10.1101/sqb.1958.023.01.012. [DOI] [PubMed] [Google Scholar]
- HOTCHKISS R. D., EVANS A. H. Fine structure of a genetically modified enzyme as revealed by relative affinities for modified substrate. Fed Proc. 1960 Dec;19:912–925. [PubMed] [Google Scholar]
- Higgins R. C., Dahmus M. E. Rapid visualization of protein bands in preparative SDS-polyacrylamide gels. Anal Biochem. 1979 Mar;93(2):257–260. doi: 10.1016/s0003-2697(79)80148-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacks S. A., Dunn J. J., Greenberg B. Identification of base mismatches recognized by the heteroduplex-DNA-repair system of Streptococcus pneumoniae. Cell. 1982 Dec;31(2 Pt 1):327–336. doi: 10.1016/0092-8674(82)90126-x. [DOI] [PubMed] [Google Scholar]
- Lacks S. A., Lopez P., Greenberg B., Espinosa M. Identification and analysis of genes for tetracycline resistance and replication functions in the broad-host-range plasmid pLS1. J Mol Biol. 1986 Dec 20;192(4):753–765. doi: 10.1016/0022-2836(86)90026-4. [DOI] [PubMed] [Google Scholar]
- Lacks S. Integration efficiency and genetic recombination in pneumococcal transformation. Genetics. 1966 Jan;53(1):207–235. doi: 10.1093/genetics/53.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez P., Espinosa M., Lacks S. A. Physical structure and genetic expression of the sulfonamide-resistance plasmid pLS80 and its derivatives in Streptococcus pneumoniae and Bacillus subtilis. Mol Gen Genet. 1984;195(3):403–410. doi: 10.1007/BF00341440. [DOI] [PubMed] [Google Scholar]
- Martinez S., Lopez P., Espinosa M., Lacks S. A. Cloning of a gene encoding a DNA polymerase-exonuclease of Streptococcus pneumoniae. Gene. 1986;44(1):79–88. doi: 10.1016/0378-1119(86)90045-4. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Ortiz P. J. Dihydrofolate and dihydropteroate synthesis by partially purified enzynes from wild-type and sulfonamide-resistant pneumonococcus. Biochemistry. 1970 Jan 20;9(2):355–361. doi: 10.1021/bi00804a024. [DOI] [PubMed] [Google Scholar]
- Ortiz P. J., Hotchkiss R. D. The enzymatic synthesis of dihydrofolate and dihydropteroate in cell-free preparations from wild-type and sulfonamide-resistant pneumococcus. Biochemistry. 1966 Jan;5(1):67–74. doi: 10.1021/bi00865a010. [DOI] [PubMed] [Google Scholar]
- Reeve J. N., Mendelson N. H., Coyne S. I., Hallock L. L., Cole R. M. Minicells of Bacillus subtilis. J Bacteriol. 1973 May;114(2):860–873. doi: 10.1128/jb.114.2.860-873.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richey D. P., Brown G. M. The biosynthesis of folic acid. IX. Purification and properties of the enzymes required for the formation of dihydropteroic acid. J Biol Chem. 1969 Mar 25;244(6):1582–1592. [PubMed] [Google Scholar]
- Roland S., Ferone R., Harvey R. J., Styles V. L., Morrison R. W. The characteristics and significance of sulfonamides as substrates for Escherichia coli dihydropteroate synthase. J Biol Chem. 1979 Oct 25;254(20):10337–10345. [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- SHIOTA T., DISRAELY M. N., MCCANN M. P. THE ENZYMATIC SYNTHESIS OF FOLATE-LIKE COMPOUNDS FROM HYDROXYMETHYLDIHYDROPTERIDINE PYROPHOSPHATE. J Biol Chem. 1964 Jul;239:2259–2266. [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Shiota T., Baugh C. M., Jackson R., Dillard R. The enzymatic synthesis of hydroxymethyldihydropteridine pyrophosphate and dihydrofolate. Biochemistry. 1969 Dec;8(12):5022–5028. doi: 10.1021/bi00840a052. [DOI] [PubMed] [Google Scholar]
- Stassi D. L., Lopez P., Espinosa M., Lacks S. A. Cloning of chromosomal genes in Streptococcus pneumoniae. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7028–7032. doi: 10.1073/pnas.78.11.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Swedberg G., Sköld O. Characterization of different plasmid-borne dihydropteroate synthases mediating bacterial resistance to sulfonamides. J Bacteriol. 1980 Apr;142(1):1–7. doi: 10.1128/jb.142.1.1-7.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLF B., HOTCHKISS R. D. Genetically modified folic acid synthesizing enzymes of pneumococcus. Biochemistry. 1963 Jan-Feb;2:145–150. doi: 10.1021/bi00901a026. [DOI] [PubMed] [Google Scholar]