Abstract
The small GTPase Sec4p is required for vesicular transport at the post-Golgi stage of yeast secretion. Here we present evidence that mutations in SEC2, itself an essential gene that acts at the same stage of the secretory pathway, cause Sec4p to mislocalize as a result of a random rather than a polarized accumulation of vesicles. Sec2p and Sec4p interact directly, with the nucleotide-free conformation of Sec4p being the preferred state for interaction with Sec2p. Sec2p functions as an exchange protein, catalyzing the dissociation of GDP from Sec4 and promoting the binding of GTP. We propose that Sec2p functions to couple the activation of Sec4p to the polarized delivery of vesicles to the site of exocytosis.
Polarity is a general principle of cell organization that forms the basis for processes as diverse as the growth of the yeast Saccharomyces cerevisiae and synaptic secretion of transmitters from neurons in animals. Cells become polarized when functionally specialized domains are created to support a particular cell function. S. cerevisiae grow in a strongly polarized fashion. As a bud forms on one pole of the cell, it enlarges until the resulting daughter cell divides from the mother cell by formation of a septum. The establishment of polarity in these cells is the result of a complex cascade of cellular events. The selection of the bud site, its assembly, and the subsequent propagation of signals that direct the secretory apparatus are mediated by components of the cytoskeleton, including actin and septins, GTP-binding proteins, and other cytosolic factors (Govindan and Novick, 1995; for review see Drubin and Nelson, 1996). Asymmetric growth is the result of the polarized insertion of secretory vesicles into the plasma membrane. As the yeast cell cycle proceeds, the secretory apparatus and cytoskeleton are directed first towards the tip of the bud and to the neck, at the intersection between mother and daughter cells before cytokinesis (Preuss et al., 1992). Membrane traffic to the plasma membrane of budding yeast thus presents an interesting example of cell polarity.
The secretory pathway consists of sequential transport steps that use small specialized carrier vesicles. Each step requires formation of vesicles from a donor compartment, transport, docking at the target membrane, and fusion of the carrier vesicles. A large number of molecular components required for these processes in yeast have been identified by genetic screening. The Golgi to plasma membrane stage of transport involves 10 SEC genes (Novick et al., 1980), 2 SNC genes (SNC1, SNC2; Protopopov et al., 1993), and 2 SSO genes (SSO1, SSO2; Aalto et al., 1993). The gene product of SEC4 belongs to a family of small GTPases, the Sec4/Ypt/Rab proteins, that are essential for vesicular traffic. As it was first shown for Sec4p, these proteins function by cycling between a GDP-bound and a GTP-bound conformation (Novick et al., 1988; Walworth et al., 1989). The current hypotheses are that the conformational switch associated with nucleotide exchange and hydrolysis mediates vectorial transport of vesicles or regulates vesicle docking and fusion, or both (Bourne et al., 1990; Novick and Brennwald, 1993; Zerial and Stenmark, 1993; Peränen et al., 1996). Sec4p has been proposed to localize in the cell to secretory vesicles, the plasma membrane, or cytosol, depending on its nucleotide state (Goud et al., 1988; Walworth et al., 1989, 1992). The understanding of how this cycle is regulated should elucidate the function of Sec4p as well as its relationship to the other components of the vesicle transport and fusion machinery.
6 of the 10 late-acting SEC genes encode subunits of a multiprotein complex (TerBush et al., 1996) that might act downstream of Sec4p in vesicle docking (TerBush et al., 1995). However, there is so far no evidence that this complex is the immediate downstream effector of Sec4p. Upon activation by a GTPase-activating protein, Sec4p hydrolyzes its bound GTP to GDP (Walworth et al., 1992). Gdi1p, the yeast Rab GDP dissociation inhibitor, recycles Sec4p-GDP from the plasma membrane through the cytoplasm back to the Golgi complex or vesicle membrane (Garrett et al., 1994). This process may be facilitated by Dss4p (dominant suppressor of Sec4p), a nucleotide release factor that stimulates the dissociation of GDP from Sec4p (Moya et al., 1993). However, the mechanistic details that lead from Sec4p–Gdi1p in the cytoplasm to active Sec4p– GTP on secretory vesicles are still obscure, as is the role that Sec4p activation plays in the pathway that ultimately leads to membrane fusion (Novick and Brennwald, 1993).
In the present study, we have defined requirements for the polarized concentration of Sec4p at the bud tip. We demonstrate that SEC2, an essential gene that is required specifically for post-Golgi vesicular traffic, plays a key role in this process. Wild-type and other post-Golgi blocked secretion (sec) mutants show a prominent localization of Sec4p in the bud that is absent in sec2 mutants. Analysis of the phenotype of these mutants revealed that this is caused by a random accumulation of vesicles carrying Sec4p on their membrane. Our studies lead to the conclusion that Sec2p acts in Golgi to plasma membrane traffic at an earlier step than the majority of the other SEC gene products that are implicated at this stage. Furthermore, we find that Sec2p interacts directly with Sec4p to promote nucleotide exchange. We hypothesize that polarized vesicle delivery may be coupled to the activation of Sec4p in a Sec2p- dependent fashion.
Materials and Methods
Yeast Strains, Media, and Reagents
The genotypes of the Saccharomyces cerevisiae strains used in this study are listed in Table I. Cells were grown in YP medium containing 1% Bacto-yeast extract and 2% Bacto-peptone (Difco Laboratories Inc., Detroit, MI) with 2% glucose (YPD). The cell density was determined by measuring the absorbance of cell suspensions at 600 nm (model No. 4054 spectrophotometer; Pharmacia LKB Biotechnology, Piscataway, NJ). Transformations were performed by the lithium acetate method (Gietz and Schiestl, 1991). For the selection of transformants, SD medium was used (0.67% nitrogen base, 2% glucose, and complete amino acid supplements as appropriate). Yeast strain Y190 was used for the two-hybrid system (MAT a ura3-52, his3Δ200, ade2-101, lys2-801, trp1-901, leu2-3,112, gal4-542, gal80-538, URA3::GAL-LacZ, LYS2::GAL-HIS3, cyhr).
Table I.
Relevant Yeast Strains
| Strain | Genotype | |
|---|---|---|
| NY8 | MATα, ura3-52, sec1-1 | |
| NY10 | MATα, ura3-52 | |
| NY13 | MAT a , ura3-52 | |
| NY17 | MAT a , ura3-52, sec6-4 | |
| NY27 | MATα, ura3-52, sec2-59 | |
| NY28 | MATα, ura3-52, sec4-8 | |
| NY30 | MATα, ura3-52, sec5-24 | |
| NY39 | MATα, ura3-52, sec15-1 | |
| NY45 | MATα, ura3-52, sec3-2 | |
| NY132 | MATα, ura3-52, his4-619, sec2-41 | |
| NY416 | MATα, ura3-52, sec16-2 | |
| NY278 | MATα, ura3-52, act1-3 | |
| NY738 | MAT a , ura3-52, sec12-4 | |
| NY779 | MATα, leu2-3,112, sec6-4 | |
| NY1005 | MATα, ura3-52, leu2-3,112, myo2-66 | |
| NY1282 | MAT a , ura3-52, bos1-1 | |
| NY1294 | MAT a , leu2-3,112, sec6-4 | |
| NY1529 | MATα, ura3-52, sec2-78 | |
| NY1530 | MATα, ura3-52, leu2-3,113, sec2-78, 6-4 | |
| NY1535 | MATα, ura3-52, pNB170 (SEC4, URA3) | |
| NY1536 | MATα, ura3-52, pNB170 (SEC4, URA3) |
Cycloheximide, sodium azide, α factor, PMSF, leupeptin, aprotinin, chymostatin, and antipain were obtained from Sigma Chemical Co. (St. Louis, MO). Pepstatin A was from Boehringer Mannheim (Indianapolis, IN), Tween 20 was from American Bioanalytical (Natick, MA), and chemicals for SDS-PAGE were from BioRad Laboratories (Richmond, CA). The embedding media for EM, LR white resin, and SPURR were purchased from Polysciences, Inc. (Warrington, PA). 125I-protein A and ECL Western blotting detection reagent were obtained from Amersham Corp. (Arlington Heights, IL), and Zymolyase 100-T was from ICN Pharmaceuticals, Inc. (Costa Mesa, CA).
Microbiological Techniques and Plasmid Constructs
General molecular biological methods were as described (Sambrook et al., 1989). Bacterial Escherichia coli strains TG1 and DH5α were used for subcloning purposes, and recombinant proteins were produced in the BL21 strain.
Fusion constructs with the GAL4 DNA-binding domain (plasmid pAS1-CYH2) and with the GAL4 DNA activation domain (plasmid pACTII) were obtained by in frame ligation of PCR products.
Immunofluorescence Microscopy
Yeast strains were grown overnight at 25°C in YPD. The final cell density (A600) was between 0.5 and 1.0 U. The cells were then either immediately fixed (for experiments at permissive temperature) or shifted for the indicated period of time to 37°C (restrictive temperature) and fixed after the shift was completed. In some experiments, either cycloheximide (0.35 mM) or sodium azide (10 mM) was added directly to the media. The cells were fixed, transferred to isotonic media, and the cell walls removed essentially as described in TerBush and Novick (1995). After attachment to eight-well microslides (Carlson Scientific, Peotone, IL), the spheroplasts were treated with 0.5% SDS in 0.1 M Hepes, pH 7.4, and 1.0 M sorbitol for 5 min. The cells were then washed nine times with 20 μl per well of PBS containing 5 mg/ml BSA, 5 mg/ml bovine gelatin, 5 mg/ml fish skin gelatin, and 0.5% Tween 20 (PBT buffer), and nonspecific binding was blocked for 30 min in the same buffer. The incubation with 20 μl of mAb C1.2.3. against Sec4p was performed overnight at 4°C. After nine washes in PBT buffer, the cells were incubated for 1 h at room temperature with 20 μl of Texas red–conjugated donkey anti–mouse antibody. The wells were washed nine times with PBT buffer and three times with PBS containing 5 mg/ml BSA and 0.5% Tween 20. The slides were then mounted with 70% glycerol in 12 mM phosphate buffer, pH 7.5. The indirect immunofluorescence was visualized with a microscope (BMAX-50; Olympus Optical Co., Tokyo, Japan) equipped with epifluorescence using a 100× objective.
Thin-Section EM
The cells were grown overnight in YPD medium to an A600 of 1.0. For all experiments, the cultures were synchronized. An aliquot of cells that equals 4.4 OD U was harvested and incubated in 20 ml of YPD, pH 4.0, containing 1 μM α factor for 4 h at 25°C to arrest cells in G1 before the induction of small buds. For the indicated times (see Results), the cells were shifted to the restrictive temperature and then prepared for thin sectioning and EM, as described in Govindan et al. (1995). Instead of embedding in 2% agar, the cells were dehydrated and embedded as a pellet on the bottom of a microcentrifuge tube. Thin sections were cut onto Formvar-coated 100 mesh nickel grids (Electron Microscopy Sciences, Fort Washington, PA), contrasted with lead citrate and uranyl acetate, and then analyzed on an electron microscope (CM10; Philips Technologies, Cheshire, CT) at 80 kV.
Immunoelectron Microscopy
A 100-ml culture of cells was grown overnight in YPD, and the cells were harvested immediately or after a 30-min shift to 37°C by vacuum filtration using a 0.45-μm nitrocellulose membrane. The fixation of the cells and processing for immunoelectron microscopy was performed according to the method of Mulholland et al. (1994) with minor modifications. The cells were fixed in 3% formaldehyde (40 mM potassium phosphate, pH 6.7, 0.8 M sorbitol, 1 mM MgCl2, 1 mM CaCl2) without the addition of glutaraldehyde. After a 1-h incubation at room temperature, the cells were washed as decribed by Mulholland et al. (1994), starting with 50 mM sorbitol in 40 mM potassium phosphate, pH 6.7, instead of 75 mM sorbitol. The dehydration and embedding in LR white resin was done as described by Mulholland et al. (1994). Thin sections were cut and transferred onto uncoated 400 mesh nickel grids (Electron Microscopy Sciences).
Immunolabeling was performed at 25°C by directly incubating the sections on grids. First, the sections were incubated for 5 min with 0.15% glycine in PBS (140 mM NaCl, 3 mM KCl, 8 mM Na2HPO4, 1.5 mM KH2PO4) and for 10 min with 2% ovalbumin in PBST (PBS containing 0.05% Tween 20). Affinity-purified antibody against Sec4p and 10 nm colloidal gold-conjugated protein A (purchased from Dr. J. Slot, Utrecht University, School of Medicine, Utrecht, The Netherlands) were diluted in PBST with 2% ovalbumin (Sigma Chemical Co.). Incubations were for 1 h with the primary antibody and 15 min with colloidal gold-conjugated protein A. Between incubations, the grids were washed extensively with PBST. Postfixation and poststaining on the grids were essentially performed as described by Mullholland et al. (1994); the only modification was that poststaining (2% aqueous uranyl acetate) was for only 3 min instead of 1 h.
Cell Fractionation and Immunoblot Analysis
Cells (between 100 and 200 OD600 U) were grown overnight in YPD at 25°C and then transferred for 2 h to 37°C. After washing once with 10 mM NaN3 in 50 mM Tris/HCl, pH 7.5, the cells were resuspended in spheroplast media (50 mM Tris, pH 7.5, 10 mM NaN3, 1.4 M sorbitol, 40 mM β-mercaptoethanol, 0.125 mg/ml zymolyase-100T) and incubated with occasional shaking at 37°C for 45 min. Spheroplasts were pelleted, cooled on ice, and resuspended in 1 ml ice-cold lysis buffer (0.8 M sorbitol in 10 mM triethanolamine, 1 mM EDTA, pH 7.2) containing protease inhibitors (1 mM PMSF, 1 μg/ml leupeptin and chymostatin, 2.5 μg/ml pepstatin A and antipain, and 5 μg/ml aprotinin). The subcellular fractionation procedure was performed at 4°C. The suspension was homogenized 30 times in a small clearance 2-ml glass Teflon homogenizer (B. Braun Biotech Inc., Allentown, PA) and was centrifuged at 450 g for 3 min. The pellet was resuspended after adding 0.5 ml lysis buffer, homogenized (20 strokes), and centrifuged as decribed above. The supernatants (S1) were pooled, and the pellet (P1) was resuspended with lysis buffer to a final volume of 1 ml. Then 15 μl of 1 M Hepes, pH 7.2, was added to S1, and the supernatant was spun at 10,000 g for 5 min. The pellet (P2) was resuspended to a final volume of 1 ml. The supernatant (S2) was spun at 100,000 g for 15 min to obtain the S3 supernatant, and the P3 pellet that was resuspended in 1 ml lysis buffer for immediate analysis or 1 ml 0.32 M sorbitol in lysis buffer for sucrose density gradient fractionation. P3 was loaded on top of a continuous 1.75–0.4 M sucrose gradient in 5 mM Hepes, pH 7.2, that was generated with a Gradient Mate (model 117; BioComp Instruments, Inc., New Brunswick, Canada). The gradients were centrifuged for 16 h at 170,000 g in an SW41 rotor (Beckman Instruments, Inc., Fullerton, CA). 0.75-ml fractions were collected from the bottom, and the pellets were resuspended in an identical volume of lysis buffer (fraction 1). For analysis of the resulting fractions from both differential and density gradient centrifugation, equal aliquots were loaded onto SDS-PAGE gels.
Interaction Assays Using the Yeast Two-Hybrid System
S. cerevisiae Y190 strain was simultaneously transformed with DNA activation and binding domain constructs, and after growing for 3–4 d, transformants were monitored for expression of β-galactosidase activity using a 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal) filter lift assay. Briefly, yeast colonies were transferred to filter paper (No. 1; Whatman Inc., Clifton, NJ), permeabilized by submersion in liquid nitrogen for 10 s, and then laid onto a second filter that had been presoaked in Z buffer (100 mM sodium phosphate, 10 mM potassium chloride, 1 mM magnesium sulphate) containing 38 mM β-mercaptoethanol and 0.35 mg of X-Gal/ml. The filters were then incubated at 30°C for 1–10 h.
In Vitro Translation
In vitro transcription-coupled translation was performed from either the open reading frame of the protein inserted in pGEM-T or from PCR products where the 5′ primer contained a T7 promoter. The T7 polymerase TNT system of Promega Biotech (Madison, WI) was used following a protocol suggested by the distributor. RNAse inhibitor (1 U/ml; Promega) was added to all reactions. 35S-labeled Promix (10 Ci/ml; Amersham Corp.) was added for radioactive labeling to a final concentration of 1 μCi/ml. Reaction time was 90 min. Translated products were checked by SDS-PAGE gel analysis followed by autoradiography.
In Vitro Binding Assays
MBP-Sec2-59 protein was constructed by ligating sec2-59 coding DNA into pMAL-c2 (New England Biolabs, Inc., Beverly, MA). Amylose beads bound to 4 μg of maltose binding protein (MBP)1 or MBP fusion protein were mixed with 5 μl 35S-labeled, in vitro–translated Sec4p in a total volume of 500 μl. After incubation for 30 min at 30°C, the resin was washed, and bound products were separated by SDS-PAGE. Sec4p binding was analyzed by autoradiography. Input was directly loaded into the well and represents 40% of the 35S-labeled protein used in each binding reaction.
The endogenous nucleotide was removed by incubation in the presence of 10 mM EDTA, followed by gel filtration to separate nucleotide. The protein was then incubated with either 1 mM GDP, 0.5 mM GTPγS, or was left nucleotide free for 20 min at 30°C before the addition of 20 mM MgCl2. The proteins treated in this fashion were used immediately for binding assays because of the limited stability of the nucleotide-free protein.
Nucleotide Displacement and Exchange Assays
GDP displacement activity was monitored essentially as described by Roberts et al. (1995) by preloading Sec4p with [3H]GDP. Reactions were initiated by the addition of 0.5 mM GDP and either His6–Sec2-59 protein or control buffer. Sec2p was NH2-terminally tagged with six histidine residues and expressed in pET15b (Novagen, Inc., Madison, WI). Recombinant fusion protein was induced for 2 h with 0.5 mM isopropyl-β-D-thiogalactopyranoside (IPTG) before purification on NTA-agarose resin (Qiagen, Inc., Chatsworth, CA), following the protocol suggested by the manufacturer. The stated protein concentrations of Sec2-59p respresent Bradford determinations of the purified material. After incubation at 14°C, aliquots were removed at the times indicated, and the reactions were stopped by dilution into ice-cold Hepes-S100 buffer consisting of 64 mM Hepes-NaOH, pH 8.0, 100 mM NaCl, 8 mM MgCl2, 2 mM EDTA, and 0.2 mM DTT. Protein-bound radioactivity was monitored by filter binding followed by scintillation counting. Nucleotide exchange was carried out under standard conditions. Sec4p was incubated with either His6-Sec2p or control buffer in the presence of [35S]GTPγS at 14°C. Final concentrations were 5 μg/ml Sec4p and 40 μM [35S]GTPγS at 2 μCi/ml. Aliquots were removed at the times indicated, and the reactions were stopped by dilution into ice-cold Hepes-S100 buffer consisting of 64 mM Hepes-NaOH, pH 8.0, 100 mM NaCl, 8 mM MgCl2, 2 mM EDTA, and 0.2 mM DTT. Sec4p association radioactivity was monitored by filter binding followed by scintillation counting.
Results
In Wild-type Cells, Sec4p Localizes to Vesicles Concentrated in the Bud
In wild-type yeast, Sec4p is localized to the bud tip as a bright fluorescent spot (Novick and Brennwald, 1993; Fig. 1). The bud tip represents the site of exocytosis and the most active insertion of newly synthesized plasma membrane components in small budded cells. As the cell cycle proceeds into cytokinesis, new membrane components are inserted at the neck area, and a concentration of Sec4p immunofluorescence labeling is observed at the neck (data not shown). Immunoelectron microscopy demonstrates that Sec4p labeling of the bud tip reflects a concentration of secretory vesicles carrying Sec4p on their membrane at this pole of the cell (Fig. 2 C). For this experiment, cells were shifted to 37°C for 30 min and embedded followed by immunogold labeling for EM.
Figure 1.
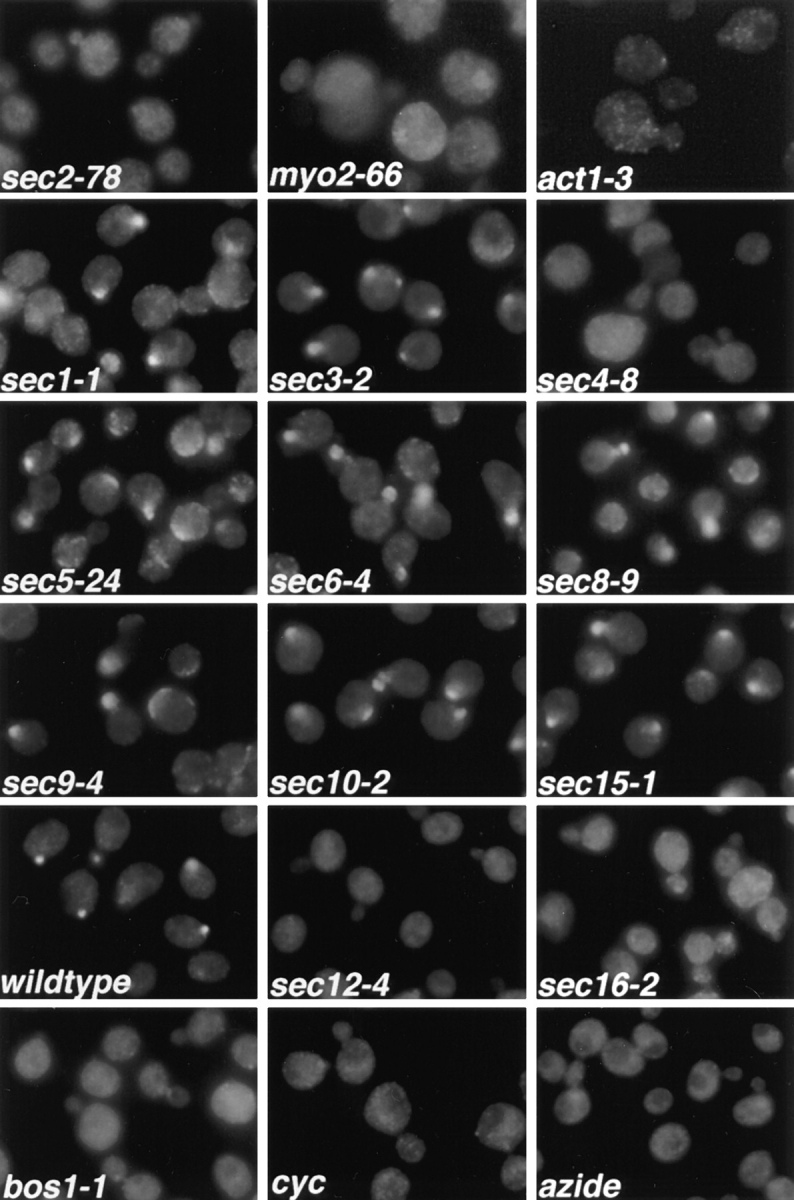
Immunofluorescence localization of Sec4p in sec mutants. Cells were grown overnight in YP dextrose medium at 25°C and then shifted to 37°C for 1 h, fixed, and labeled with αSec4 antibody. The effect of cycloheximide (cyc) and azide treatment on wild-type cells was tested by adding either agent to the culture immediately before shift to 37°C. sec2-78, NY1529; myo2-66, NY1005; act1-3, NY278; sec1-1, NY8; sec3-2, NY45; sec4-8, NY28; sec5-24, NY30; sec6-4, NY18; sec8-9, NY43; sec9-4, NY32; sec10-2, NY36; sec15-1, NY39; wild-type, NY10; sec12-4, NY738; sec16-2, NY416; bos1-1, NY1282 (see Table I for strain genotypes). Wild-type cells and most late-acting sec mutants show immunolabeling preferentially in the bud. In sec2-78, myo2-66, and act1-3, diffuse staining for Sec4p is observed. Also, in sec4-8, ER to Golgi sec mutants (sec12-4, sec16-2, bos1-1), and after treatment of wild-type cells with cycloheximide or azide, no concentration of immunolabeling in the bud is found.
Figure 2.
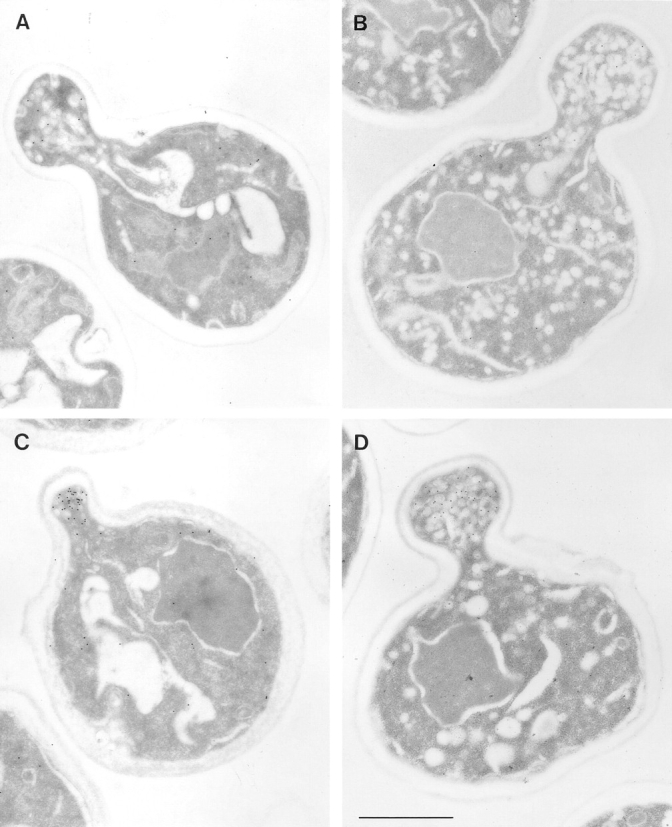
Electron microscopic analysis of sec2-78, wild-type, and sec6-4 cells labeled for Sec4p with immunogold. Cells were grown overnight at 25°C, shifted to 37°C for 30 min, and then prepared for postembedding immunogold labeling as described in Materials and Methods. (A) A sec2-78 (NY1529) cell grown at the permissive temperature where some immunogold labeling is found on vesicles in the bud. Both wild-type (NY10, C) and sec6-4 (NY1294, D) cells show a concentration of immunogold labeling on vesicles in the bud after shift to the restrictive temperature. In contrast, an average sec2-78 cell in B has immunogold-labeled vesicles randomly distributed in the bud and mother cell. At 25°C (A) 3 ± 2 gold particles were observed on vesicles while 3 ± 2 gold particles were found independent of vesicles in the bud. In the mother cell were 1 ± 1 gold particle on vesicles and 10 ± 3 gold particles not on vesicles. At 37°C (B) we found 3 ± 2 gold particles on vesicles and 3 ± 4 gold particles not on vesicles in the bud. In the mother cell were 4 ± 3 gold particles on vesicles and 15 ± 9 gold particles not on vesicles. The error represents the SD for n = 12 at 25°C and n = 24 at 37°C. Bar, 1 μm.
We find that the normal generation of Golgi-derived vesicles is required for the concentration of Sec4p immunoreactivity in the bud. We examined the Sec4p immunofluorescence localization in mutants blocked at earlier (ER to Golgi and intra-Golgi) stages of the secretory pathway. All mutants tested (sec7-1, sec12-4, sec13-1, sec14-3, sec16-2, sec20-1, sec21-1, sec22-3, sec23-1, ypt1-1, bos1-1, and bet2-1) showed a loss of bud tip labeling for Sec4p, as displayed in Fig. 1 for the examples of sec12-4, sec 16-2 (blocked in vesicle budding from the ER; Novick et al., 1980; Kaiser and Schekman, 1990), and bos1-1 (blocked in fusion of vesicles to the Golgi; Lian and Ferro-Novick, 1993). Sec4p redistribution is rapid, as can be seen in Fig. 3, which shows the Sec4p immunofluorescence in sec12-4 cells after only a 10-min shift to the restrictive temperature. These results indicate that any early block in the secretory pathway that prevents the normal formation of post-Golgi vesicles results in a redistribution of the Sec4p immunolabeling. Similarly, we found that ATP depletion by addition of azide, or a block in protein synthesis by addition of cycloheximide, resulted in a complete loss of Sec4p immunostaining in bud tips (Fig. 1). This loss of bud tip staining presumably reflects the fact that vesicle production is necessary for the normal Sec4p bud localization.
Figure 3.
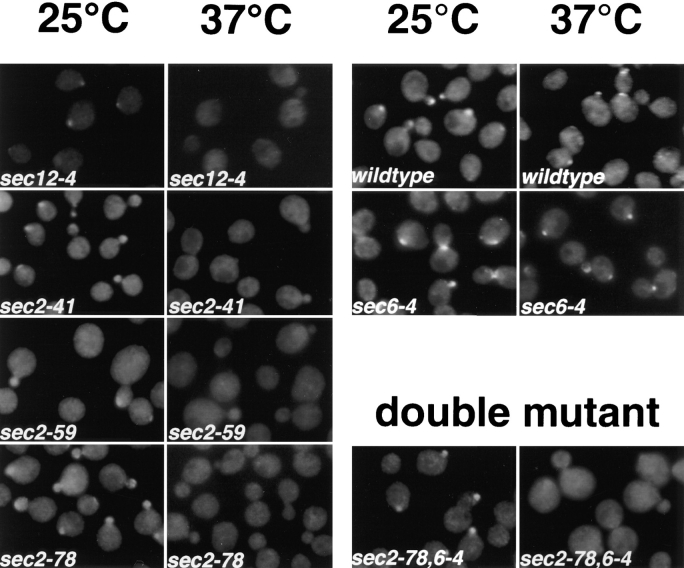
Immunofluorescence localization of Sec4p in different sec2 alleles and a sec2-78,6-4 double mutant after a short shift to the restrictive temperature. Cells were grown overnight in YP dextrose medium at 25°C and then shifted to 37°C for 10 min, fixed, and labeled with αSec4 antibody. sec12-4, NY738; sec2-41, NY132; sec2-59, NY27; sec2-78, NY1529; wild-type, NY10; sec6-4, NY779; sec2-78,6-4, NY1530. In sec2-41, sec2-59, and sec2-78, Sec4p, immunostaining was concentrated in the bud at 25°C but was diffuse already after 10-min shift to the restrictive temperature. In the ER-to-Golgi mutant sec12-4, diffuse immunolabeling was also already observed after 10 min at 37°C. Both in wild-type and sec6-4, Sec4p immunolabeling was concentrated at the bud both at 25°C and after 10 min at 37°C. The double mutant was constructed as described in Materials and Methods. sec2-78, sec2-78,6-4 shows labeling in the buds at the permissive temperature that is no longer detected after 10 min at the restrictive temperature.
The effect of cycloheximide or azide on the Sec4p immunolocalization observed in wild-type cells after 1 h at 37°C (Fig. 1) was also found after only 10 min (data not shown). Prolonged incubation of wild-type cells at 4°C did not affect the localization of Sec4p (data not shown). Although cycloheximide treatment of wild-type cells at 37°C resulted in a complete redistribution of Sec4p, simultaneous blocks of protein synthesis and vesicle traffic by addition of cycloheximide and incubation at 4°C, did not abolish bud tip immunostaining for Sec4p (data not shown).
In summary, the slowing of membrane traffic at 4°C does not affect the localization of Sec4p. A block in vesicle generation by the addition of cycloheximide does not result in Sec4p mislocalization at 4°C, probably because the low temperature slows vesicle fusion so that vesicles are still present in the bud tip. However, if fusion is allowed to occur at a normal rate, a block in vesicle generation imposed by the addition of cycloheximide or azide or by a sec mutant block results in the loss of localization of Sec4p to the bud tips.
Sec4p Is Redistributed in sec2-78, myo2-66, and act1-3, but Not in Other Post-Golgi Mutants
In a search for factors involved in Sec4p localization, we studied the immunolocalization of Sec4p in temperature-sensitive sec mutants blocked at the post-Golgi stage of the secretory pathway. Immunofluorescence labeling with anti-Sec4p antibodies was performed after a 1-h shift of the cells to the restrictive temperature (Fig. 1). In the majority of the post-Golgi sec mutants (sec1-1, sec3-2, sec5-24, sec6-4, sec8-9, sec9-4, sec10-2, and sec15-1; see Fig. 1), a concentration of the immunofluorescence labeling of Sec4p in the bud tip was still observed. Sec6-4 was chosen as a representative of this group for analysis by immunoelectron microscopy (Fig. 2 D). We found strong labeling of Sec4p on vesicles concentrated at the bud tip after a shift to 37°C for 30 min. Even though vesicles start to build up in the mother cell after 30 min at the restrictive temperature, vesicles at or near the bud tip appear to be much more highly labeled for Sec4p. This localization of Sec4p indicates that in this group of mutants, the block in the secretory pathway occurs after Sec4p has bound to the vesicle membrane and the vesicles have concentrated at the bud tip. The enlargement of the fluorescent spot in these sec mutants compared to wild-type cells (Fig. 1) probably results from the accumulation of vesicles in the bud tip at the restrictive temperature.
In three of the examined vesicle-accumulating mutants, namely sec2-78, myo2-66, and act1-3, a striking redistribution of the Sec4p immunofluorescence labeling was observed (Fig. 1). The concentration in the bud was lost and, instead, a rather diffuse labeling throughout the cell was observed. In sec2-78, the labeling was most evenly distributed, while in myo2-66, bud tips appeared less labeled than the mother cell, and the labeling was somewhat punctate. Finally, in act1-3, the Sec4p immunofluorescence labeling appeared punctate throughout the whole cell. In addition, all three mutants show some enlargement of the cells after a long shift to 37°C. This is more prominent in myo2-66 and act1-3, but it is also observed in sec2-78.
The myo2-66 mutant has been previously studied in detail. Unlike sec1-1 and sec6-4 mutants that accumulate vesicles first at their bud tip, in myo2-66, vesicles primarily accumulate in the mother cell and not in the bud (Govindan et al., 1995). The observed Sec4p immunofluorescence parallels this pattern of vesicle accumulation and is consistent with the proposed role of Myo2p in polarized vesicle delivery (Govindan et al., 1995).
Conditional lethal mutations in the essential gene encoding actin in yeast (ACT1) have been shown to result in the accumulation of invertase-containing secretory vesicles and a loss of polarity that becomes apparent as a reduction in the number of budded cells and delocalized chitin deposition (Novick and Botstein, 1985). ACT1 is therefore thought to be required for polarized vesicle transport in yeast. As in the myo2-66 mutant, the redistribution of the Sec4p immunostaining in act1-3 mutants could result from redistribution of vesicles caused by their inefficient transport.
An abnormal staining pattern of Sec4p was also observed in sec4-8 (Fig. 1). In this mutant, Sec4p is present at much lower levels than in wild-type cells and is defective in its nucleotide binding ability on nitrocellulose blots (Goud et al., 1988; Walworth et al., 1989). Accordingly, we found a lower level of overall Sec4p immunofluorescence.
In summary, we found that normal formation of post-Golgi vesicles is required for proper immunolocalization of Sec4p. Among the genes required at the final stage of the secretory pathway, SEC2, MYO2, and ACT1 are necessary for localization of Sec4p to the tip of budding cells. Myo2p, a class V myosin (Johnston et al., 1991), and Act1p have been proposed to act in vesicle transport as components of the cytoskeletal transport machinery. However, the mechanism of action of Sec2p in secretion is, as yet, completely unknown (Nair et al., 1990). Since sec2 mutants exhibit a normal actin distribution by rhodamine– phalloidin staining (data not shown), we excluded that Sec2p alters Sec4p localization through an effect on the actin cytoskeleton. To understand the observed redistribution of the Sec4p immunolabeling in sec2-78, we decided to further characterize the phenotype of this and other sec2 mutants.
Sec4p Is Rapidly Redistributed in Different Alleles of sec2
A redistribution of the Sec4p immunolabeling was also seen in the two previously described temperature-sensitive alleles, sec2-41 and sec2-59 (Nair et al., 1990), after a 1-h shift to the restrictive temperature (data not shown). While sec2-41 and sec2-59 both express a truncated form of the protein lacking the COOH-terminal half (Nair et al., 1990), the newly isolated allele, sec2-78, produces a full-length protein (data not shown). We next asked how soon after shift to the restrictive temperature the redistribution of the Sec4p labeling occurs. In all three sec2 alleles, a diffuse Sec4p immunolabeling was already observed after a 10-min shift to 37°C (Fig. 3). Both wild-type and sec6-4 cells clearly showed Sec4p bud tip staining at this time point (Fig. 3).
Sec2p Acts Before Sec6p
The sequence of action of two genes participating in the same pathway can be established by analysis of the phenotype of a double mutant, so long as the double mutant is viable and the two single mutants have distinct phenotypes. A sec2-78, sec6-4 double mutant has a normal Sec4p distribution at the permissive temperature but shows the same redistribution of labeling after a 10-min shift to the restrictive temperature, as seen with the sec2-78 single mutant (Fig. 3). This demonstrates that Sec2p acts before Sec6p.
Sec4p in sec2 Mutants Is Still Found on Secretory Vesicles
The diffuse appearance of the Sec4p immunofluorescence in sec2 mutants could be explained in several ways: an overall decrease in the abundance of the protein, a membrane attachment defect, or a redistribution of vesicles carrying Sec4p on their membranes.
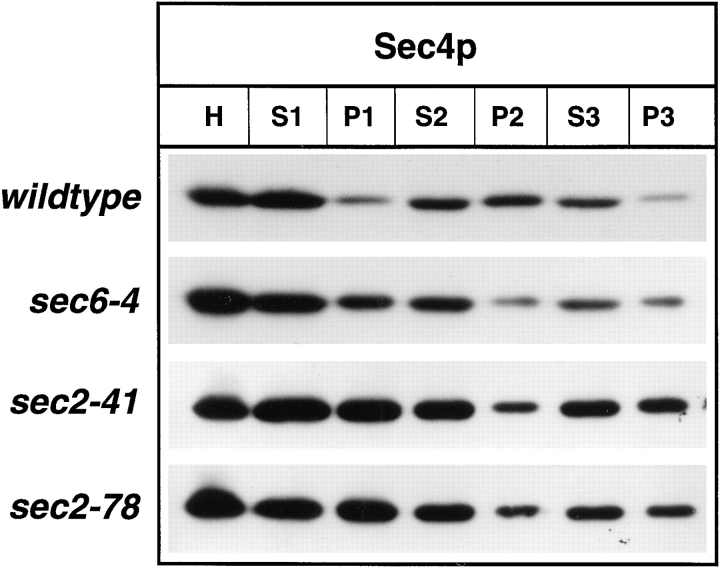
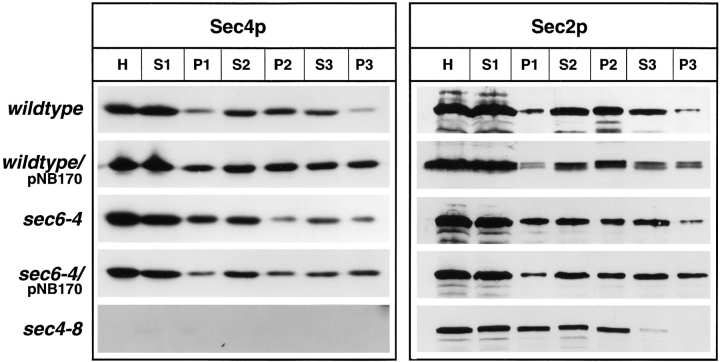
To answer this question, differential centrifugation was performed on lysates of yeast incubated at the restrictive temperature for 2 h. Upon subcellular fractionation (Fig. 4), we did not find any indication for a reduction in the abundance of Sec4p or a major shift of the protein into the soluble pool (100,000 g supernatant, S3) in either sec2-41 or sec2-78 compared to wild-type or sec6-4 cells. Previously, a shift of Sec4p from the 10,000 g pellet (P2) to the 100,000 g pellet (P3) as vesicles accumulate in sec6-4 cells has been demonstrated (Goud et al., 1988). This reflects a redistribution of Sec4p from the plasma membrane to the vesicular pool. A similar situation was observed in sec2 mutants, indicating that there is no defect in the membrane attachment of Sec4p.
Figure 4.
Differential centrifugation of lysates derived from wild-type (NY10), sec6-4 (NY779), sec2-41 (NY132), and sec2-78 (NY1529). Cells were grown overnight in YP dextrose and then shifted to 37°C. After 2 h at the restrictive temperature, cells were harvested, and lysates (S1) were prepared as described in Materials and Methods. S1 supernatants were centrifuged at 10,000 g to generate supernatant S2 and pellet P2. The S2 supernatants were centrifuged at 100,000 g to obtain supernatant S3 and pellet P3. Equal volumes of samples were prepared for electrophoresis, separated on SDS-PAGE gels, and transferred to nitrocellulose. Western blots were probed with αSec4p polyclonal antibodies and iodinated protein A. No difference in the relative distribution of Sec4p between S3 and P3 in sec2-41 and sec2-78 compared to sec6-4 is seen. The shift of Sec4p from P2 to P3 in sec6-4 compared to wild-type is caused by vesicle accumulation.
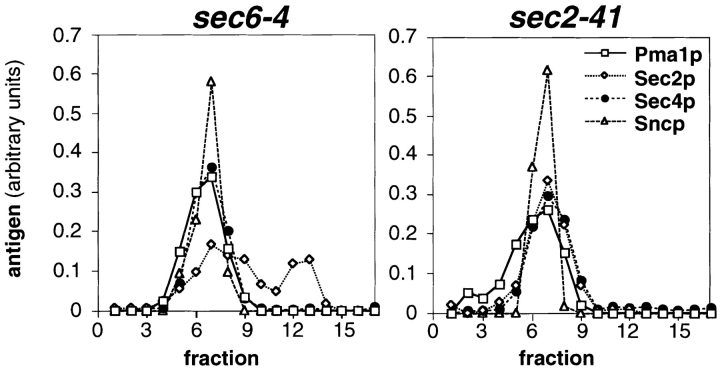
To directly assess whether Sec4p is associated with vesicle membranes in sec2 mutants, we performed subcellular fractionation using sucrose density gradients. P3 fractions derived from cells that had been shifted to the restrictive temperature were loaded on top of 0.3–1.75 M sucrose gradients and centrifuged to equilibrium. Fractions were collected from the bottom of the gradients and analyzed by Western blotting for Sec4p, the vesicle marker Sncp (Protopopov et al., 1993), and the vanadate-sensitive ATPase (Pma1p) that is found both in plasma membrane and secretory vesicles (Walworth and Novick, 1987). Sec4p cofractionated in sec2-41 cells with Sncp and Pma1p in one peak (Fig. 5), as it does in sec6-4 cells, indicating that all of the Sec4p found in P3 is bound to vesicles (Fig. 5). A similar result was obtained for sec2-78 (data not shown). Results concerning the distribution of Sec2p will be discussed in a later section.
Figure 5.
Fractionation of P3 pellets from sec6-4 and sec2-41 cells shifted to 37°C for 2 h on sucrose density gradients. The P3 pellets were generated by differential centrifugation and loaded in 0.32 M sorbitol on top of 0.4–1.75 M sucrose gradients. After centrifugation to equilibrium, the gradients were fractionated from the bottom (fraction 2) in 17 fractions (0.75 ml), and the residual pellets were resuspended in 0.75 ml (fraction 1). Equal volumes of each fraction were analyzed by SDS-PAGE and immunoblotting (affinity-purified polyclonal antibodies to Pma1p and Sec2p detected by chemoluminescence; antisera to Sec4p and Sncp detected by iodinated protein A). The Western blots were quantified by densitometry and for each antigen and gradient normalized to a total integrated optical density of 1. In sec2-41 cells (NY132), Sec4p peaks at the same density as in sec6-4 (NY779). Sec2p partially cofractionates with Sec4p and Sncp in sec6-4 cells and completely in sec2-41 cells.
In sec2 Mutants, Secretory Vesicles Accumulate both in Bud and Mother Cells
Since we saw no indication of a defect in the attachment of Sec4p to vesicle membranes in the sec2 mutants, the observed Sec4p mislocalization could simply result from a failure of the secretory vesicles to accumulate at the site of exocytosis. This question was addressed by EM.
In wild-type cells, only a few secretory vesicles are observed by EM as a result of their rapid transit to the cell surface and fusion (Fig. 6 C). In secretory mutants, it is possible to determine where vesicles first accumulate in small budded cells after a short shift to the restrictive temperature. For this experiment, sec2-78 cells were subjected to fixation, embedding, and thin-section analysis shortly after being released from G1 arrest. At the permissive temperature, few vesicles were found at the bud tip or in the mother cell (Fig. 6 A). After a shift of the cells to 37°C for 10 min, the total number of vesicles has doubled with approximately equal numbers of vesicles observed in both bud and mother cells (Fig. 6 B). Within 30 min of a shift to 37°C, relatively more vesicles were found in the mother cell than in the bud (data not shown). This pattern of vesicle accumulation is clearly different from that of a later-acting sec mutant such as sec6-4 (10 min at 37°C; Fig. 6 D). In sec6-4, vesicles first accumulate at the bud tip, and only after a prolonged shift, when the bud is filled, do vesicles accumulate in the mother cell (Govindan et al., 1995).
Figure 6.
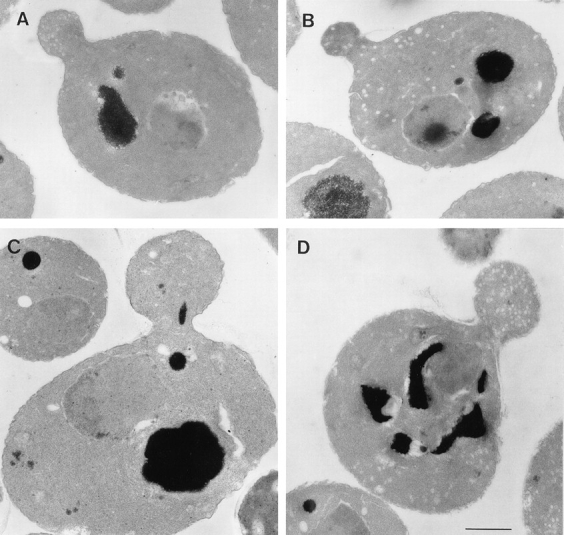
EM of sec2-78, wild-type, and sec6-4 cells shifted to 37°C for 10 min. Cells were synchronized with α factor, released from G1 arrest, and allowed to form small buds at 25°C, and then incubated at 37°C for 10 min. After this, cells were fixed and prepared for EM, as described in Materials and Methods. (A) Representative sec2-78 cell (NY1529) at 25°C that shows some vesicle accumulation. (B) Representative sec2-78 cell after 10 min at 37°C that accumulated vesicles both in bud and mother cell. In comparison, a typical wild-type cell (NY13; C) does not accumulate secretory vesicles, and in sec6-4 (NY17; D), vesicles accumulate mainly in the bud. Bar, 1 μm.
We used immunoelectron microscopy to further examine the distribution of Sec4p in sec2 mutants (Fig. 2). In sec2-78, at the permissive temperature, there is some immunogold labeling of vesicles in the bud tip, although this is decreased compared to wild-type or sec6-4 (Fig. 2 A). After a 30-min shift to the restrictive temperature, a few vesicles in both the bud and mother cell were found to be labeled (Fig. 2 B). Similar results were obtained for sec2-41 (data not shown). This Sec4p localization is very different from that in wild-type and sec6-4 cells (Fig. 2, C and D, respectively), where a concentration of vesicles with Sec4p immunolabeling is observed in the bud.
To summarize, in sec2 mutants, Sec4p binds to vesicle membranes normally, but it is redistributed as a consequence of a randomized accumulation of the secretory vesicles themselves. Therefore, we conclude that the sec2 mutant phenotype is caused by either a deficiency in vesicle targeting to the bud or retention in the bud.
Sec2p Interacts Directly with Sec4p
As a next step towards understanding the role of Sec2p, we asked whether Sec2p can bind directly to Sec4p. A yeast two-hybrid system was used to identify interactions between these two proteins (Chien et al., 1991). Gal4 DNA-binding domain fusions were constructed in pAS-CYH2. Expression of the fusion protein was confirmed by Western blot analysis with mAb 12CA5, which recognized the HA1 epitope incorporated into the fusion protein immediately adjacent to the Gal4 domain. The SEC2 construct was screened against a panel of constructs consisting of SEC4 wild-type and various point mutations of SEC4 either containing the COOH-terminal prenylation motif or not, as indicated (Table II).
Table II.
Two-hybrid Interactions between SEC2 and SEC4
| Sec4 construct | Amino acid change | Class | Sec2p | Sec2-59p | ||||
|---|---|---|---|---|---|---|---|---|
| Wild type | − | − | ||||||
| ΔC | Cys214,215→ Δ | Wild-type protein lacking | − | − | ||||
| COOH-terminal prenylation | ||||||||
| L79 | Gln79→ Leu | GTPase deficient | − | − | ||||
| V29 | Ser29→ Val | ND | + | + | ||||
| V29, ΔC | Ser29→ Val, Cys214,215→ Δ | ND | + | + | ||||
| N34, ΔC | Ser34→ Asn, Cys214,215→ Δ | Equivalent to Ras N17* | + | + | ||||
| I133, ΔC | Asn133→ Ile, Cys214,215→ Δ | Nucleotide binding deficient* | + | + |
β-Galactosidase activity was determined by filter assay. SEC4 and mutants were expressed as GAL4 DNA–binding domain fusions, and targets were expressed as GAL4 DNA activation domain fusion. Pairs were coexpressed in the yeast reporter strain Y190. Plus (+) represents a positive and minus (−) a negative indication of β-galactosidase activity. At least 10 independent transformants were tested for each pair.
Sec4ΔC contains a deletion of the COOH-terminal cysteines that are necessary for geranylgeranylation and thus, membrane attachment of the protein. Sec4N34 contains a substitution of asparagine for serine at a position that is conserved for all small GTPases and is analogous to the dominant blocking allele RasN17. In Ras, this mutant may accommodate GDP in its binding pocket, but probably adopts a structure intermediate between the GDP- and GTP-bound states (Farnsworth and Feig, 1991). GDP dissociation inhibitor (GDI) cannot interact with the Sec4N34 mutant allele, further indicating that this mutant is not in the GDP-bound conformation that would be favored for a physical interaction with GDI (data not shown). The Sec4L79 mutant has been shown to lower the intrinsic hydrolysis rate of GTP (Walworth et al., 1992), as has also been demonstrated for other Rab proteins (Stenmark et al., 1994). Sec4I133 contains an asparagine to isoleucine substitution and results in a protein unable to bind guanine nucleotides. This point mutation is a dominant negative inhibitor of membrane traffic in yeast (Walworth and Novick, 1989). The Sec4V29 mutant substitutes a valine residue for a nonconserved serine. In the case of Ras, the residue substituted is a glycine, and the mutant protein corresponds to the activated oncogenic form. In the case of Rab proteins, however, this point mutation is not well understood and may more resemble the GDP-bound conformation (Janoueix-Lerosey et al., 1995; Brondyk et al., 1995).
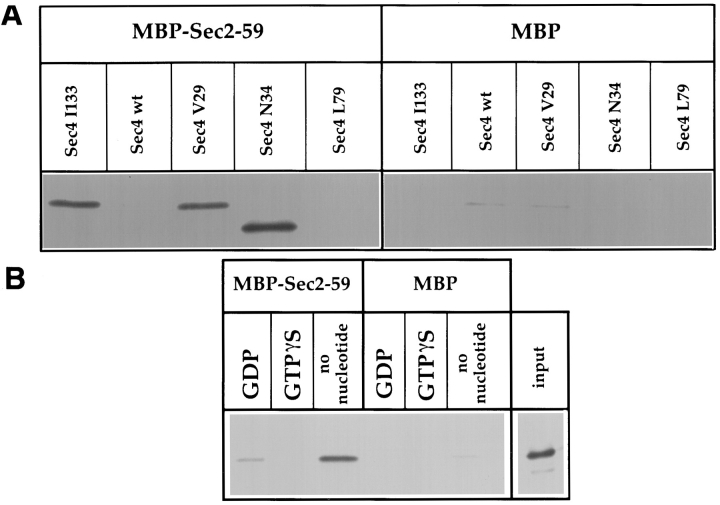
The results of the two-hybrid assays are shown in Table II. Positive interactions are indicated by β-galactosidase activity. Sec2p was found to interact with the N34, I133, and V29 alleles of SEC4, but not with the wild-type or L79 SEC4 alleles. The level of interaction was unchanged, regardless of whether the SEC4 construct was lacking the COOH-terminal prenylation motif. This indicates that the observed interaction is not dependent on the posttranslational prenylation of Sec4p, as would be required for Sec4p/Gdi1p interactions. A similar two-hybrid assay performed with GDI1 is positive with wild-type SEC4 constructs but not those lacking the COOH-terminal cysteines (Collins et al., 1997) reflecting the GDI requirement for prenylated Rab protein (Araki et al., 1991). As the COOH terminus of Sec2p is dispensable for normal function at 25°C in vivo (Nair et al., 1990), we asked whether the NH2-terminal domain alone is sufficient for Sec4p interaction. The sec2-59 construct, which lacks the COOH-terminal domain of SEC2, interacted with the SEC4 constructs in the two-hybrid assay to an extent indistinguishable from full length SEC2. The positive interactions observed between mutant forms of Sec4p and Sec2p indicate that these point mutants of SEC4 adopt a conformation that will stabilize the interaction with Sec2p.
One possible caveat for two-hybrid assays with endogenous S. cerevisiae proteins is that a positive interaction may reflect either a direct association or an indirect association mediated by a third endogenous, bridging protein. To determine whether Sec4p associates directly with Sec2p, we attempted to reconstruct the interaction in vitro using Sec2-59 recombinant MBP fusion protein and 35S-labeled Sec4 wild-type and mutant proteins generated in a coupled transcription/translation system. Unfused MBP was used as a negative control. The recombinant proteins were mixed for 30 min at 30°C, washed, and subjected to SDS-PAGE followed by autoradiography. The results are shown in Fig. 7 A, where the binding of Sec4 wild-type and mutant proteins can be directly compared. Binding of MBP–Sec2-59 can be observed for Sec4V29, Sec4N34, and Sec4I133, and no association is detected for Sec4 wild-type or Sec4L79. These results exactly parallel the data obtained in the two-hybrid assay.
Figure 7.
In vitro association of Sec2p with Sec4p. The binding assays were performed on amylose resin using recombinant MBP-Sec2-59 or MBP alone, which was incubated with 35S-labeled Sec4p produced in an in vitro transcription-coupled translation reaction. (A) The SEC4 mutations are indicated above each lane. (B) Wild-type Sec4p was diluted in buffer with EDTA before being gel filtered and associated with nucleotide as indicated. Input was directly loaded into the well and represents 40% of the 35S-labeled protein used in each binding reaction.
Sec2p Interacts with Sec4p in the Nucleotide-free Conformation
The three states of Rab nucleotide binding, GDP-bound, GTP-bound, and nucleotide-free, are known to have distinct conformations (Simon et al., 1996). Point mutations in the nucleotide-binding region may stabilize interactions with Rab accessory proteins by trapping the protein in one of these conformations. To relate the situation observed with mutant forms of Sec4p to the conformations of wild-type Sec4p, we prepared wild-type Sec4p that was bound either to GDP, GTPγS, or stripped of bound nucleotide. The in vitro–translated Sec4p was then assayed for Sec2p binding. The results are shown in Fig. 7 B. Sec2p shows no affinity for the “activated” form of Sec4p that is bound to GTPγS. A weak interaction with Sec4p-GDP can be observed; however, there is a strong preference for interaction with Sec4p that is nucleotide free, illustrated by comparing the level of input versus bound protein. These data demonstrate that Sec2p interacts preferentially with the nucleotide-free conformation of Sec4p and that the interaction is mediated by the NH2-terminal region of Sec2p.
Sec2p Is A Guanine Nucleotide Exchange Factor (GEF) for Sec4p
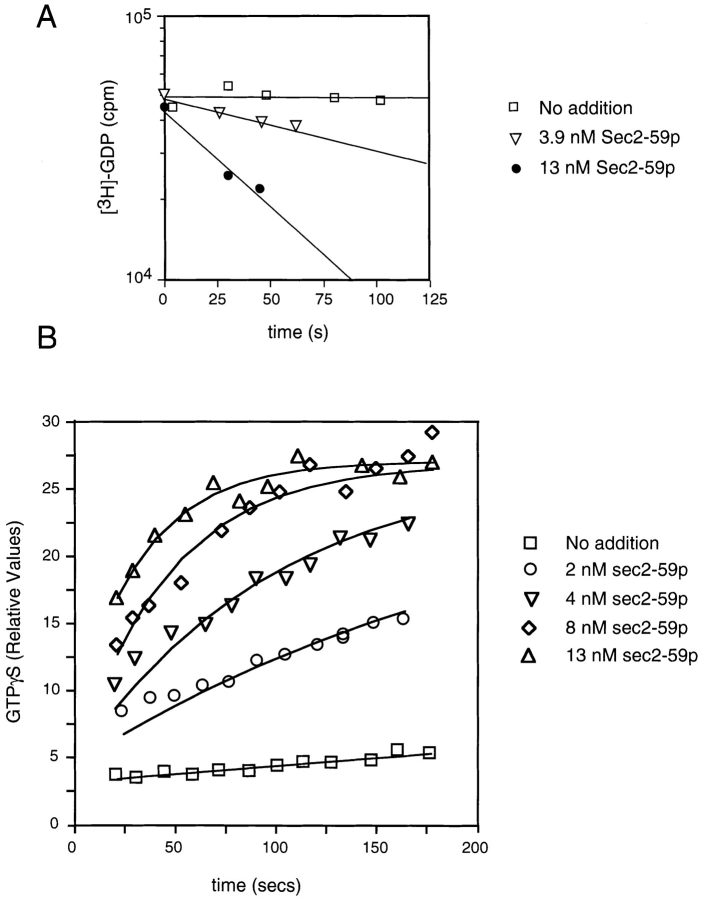
The binding characteristics of Sec2p for Sec4p suggested that Sec2p may act as a guanine nucleotide exchange protein. The ability of Sec2p to stimulate guanine nucleotide exchange on Sec4p was directly tested using recombinant proteins (Fig. 8). For these experiments, we used Sec2-59p, the truncated allele of Sec2p, since the Sec2-59p was more readily expressed in bacteria, interaction data showed that this domain was fully capable of binding Sec4p, and in vivo, sec2-59 can function as the sole copy of the gene at 25°C. These assays were performed at 14°C since Sec4p possesses a high intrinsic GDP off rate relative to other Rab proteins (Kabcenell et al., 1990; Yu and Schreiber, 1995). Sec2p has the ability to stimulate the rate of GDP displacement of Sec4p at substoichiometric levels. With 1 μM Sec4p and 3.9 nM Sec2p, an ∼15-fold stimulation of the GDP release rate is observed (Fig. 8 A). Stimulation of the rate of GTP association can also be observed (Fig. 8 B). The nonhydrolyzable analogue GTPγS was used in this experiment to avoid interference by nucleotide hydrolysis. In contrast to a recently described GEF for Rab3A (Wada et al., 1997), which requires prenylation of the substrate, Sec2p is active on recombinant Sec4p, indicating that COOH-terminal prenylation is not necessary for productive interactions.
Figure 8.
Sec2-59p–stimulated guanine nucleotide exchange on Sec4p. (A) Aliquots containing 1 μM Sec4p-[3H]GDP were incubated with the indicated levels of Sec2-59p or buffer in the presence of excess unlabeled GDP. At the time intervals indicated, Sec4p-bound radioactivity was determined by the filter-binding assay. To retard the intrinsic off rate of Sec4p, this experiment was performed at 14°C. (B) Aliquots containing 1 μM Sec4p were incubated with Sec2-59p or buffer, as indicated in the presence of [35S]GTPγS at 14°C. At the time intervals indicated, Sec4p-bound radioactivity was determined by the filter-binding assay. For each assay, the relative values correspond to the nanomoles of GTPγS retained on the filter.
A Family of Sec2p-related Sequences
Interestingly, the NH2 terminus of Sec2p, which we demonstrate to be sufficient for binding to Sec4p, exhibits a close similarity to the mammalian protein Rabin3 (Fig. 9; Brondyk et al., 1995). Rabin3 has been shown to bind the Rab protein Rab3A. Both Sec2p and Rabin3 contain NH2-terminal domains with regions predicted to assume a coiled-coil conformation. The highest stretch of sequence similarity is found immediately after the coiled-coil region. This suggests that Sec2p is the prototype of a protein family, related through a coiled-coil domain that binds to Rab proteins. Use of a consensus sequence of this region to search the GenBank/EMBL/DDBJ database (Altschul et al., 1990) revealed the existence of several other related sequences (Fig. 9). As the related sequences all represent expressed sequence tags (EST), the overall relevance of this homology cannot be ascertained at the present time. It should be noted, however, that this Sec2-like family of sequences is distinct from the coiled coil Rabaptin family of Rab-binding proteins, since Rabaptin preferentially binds the GTP-activated state of Rab (Stenmark et al., 1995).
Figure 9.
Sequence of Sec2p and comparison with Rabin3 and expressed sequence tags. Comparison of the Rab-binding portion of the protein sequence of Sec2p with Rabin3 and the predicted protein sequence of ESTs from human liver (H73617), human brain (T09468/N24211), and Caenorhabditis elegans (D37628) homologues. The sequences were aligned using the program MegAlign and visual inspection. EST sequences, which are incomplete cDNA sequences, are shown only for the predicted open reading frames corresponding to the SEC2 sequence. Amino acid residues are numbered according to the protein or DNA sequence. Gaps to improve the alignment are indicated by hyphens. Amino acids that are identical to the yeast sequence in at least two of the five sequences are shaded black. Residues that are similar in class are shaded grey and are grouped according to acidic DE, basic HKR, hydrophobic MPVWAFIL, and polar CGNQSTY.
Sec2p Association with Secretory Vesicles Is Mediated by Sec4p
Since Sec2p has a direct effect on the localization of vesicles carrying Sec4p, and since there is a direct interaction between the two proteins, the site of this interaction in the cell may be on the vesicle membrane. We examined the distribution of Sec2p on sucrose density gradients of the P3 fraction isolated from sec6-4 and sec2-41 mutant cells (Fig. 5). The small vesicular membrane fraction from sec6-4 lysates showed two peaks of Sec2p: one of them cofractionating with the peaks of Sec4p and Sncp; the other one at lower sucrose density. These data show that a pool of Sec2p associates with vesicles. Interestingly, in sec2-41, there was only one peak of Sec2p that cofractionated with Sec4p and Sncp, perhaps suggesting that the affinity of Sec2p binding on vesicles may be increased in the case of the truncated mutant protein or that absence of the COOH terminus prevents the action of a third factor that mediates interaction with a structure that migrates in a lighter fraction.
Lysates of sec4-8 mutant cells were fractionated to determine if functional Sec4p is required for vesicle association of Sec2p. Although there is an accumulation of vesicles in this mutant with a comparable peak of Sncp, there is very little Sec4p on the vesicles as a result of the instability of the mutant protein (data not shown). We could not detect any Sec2p in the P3 fraction derived from sec4-8 lysates (Fig. 10). Therefore, the presence of Sec2p in the vesicle fractions depends on the presence of Sec4p.
Figure 10.
Differential centrifugation of lysates derived from wild-type (NY10), wild-type overproducing Sec4p on low copy (NY1535), sec6-4 (NY779), sec6-4 overproducing Sec4p (NY1536), and sec4-8 (NY28). Cells were grown overnight in YP dextrose and then shifted to 37°C. After 2 h at the restrictive temperature, cells were harvested and lysates (S1) were prepared as described in Materials and Methods. Differential centrifugation and analysis of the resulting fractions was performed as described in Fig. 4. Western blots were probed with αSec4p polyclonal antibodies and iodinated protein A or affinity-purified αSec2p polyclonal antibodies detected with chemoluminescence. The amount of Sec2p in the P3 fractions parallels the amount of Sec4p. The increase of Sec4p in P3 of strains overproducing the protein is correlated with an increase of Sec2p, and the decrease of Sec4p in P3 in sec4-8 cells is correlated with a decrease of Sec2p in this fraction. The Sec4p blots for wild-type and sec6-4 cells are identical to those shown in Fig. 4 to allow for direct comparison.
Since a decrease in the amount of Sec4p on vesicles prevents Sec2p association with vesicles, we asked whether the converse effect could be seen by overexpressing Sec4p. For this experiment, pNB170, a low copy SEC4 plasmid, was introduced into wild-type, sec6-4, sec2-41, and sec2-78 cells. In wild-type and sec6-4 cells carrying pNB170, in which Sec4p is overexpressed, more Sec4p was found in 100,000 g pellets compared to untransformed controls (Fig. 10). This increase of Sec4p in P3 was paralleled by an increase in Sec2p, again stressing the correlation in the presence of the two proteins on small vesicular membranes.
Overexpression of Sec4p by pNB170 suppresses sec2-78 at 38°C (data not shown). It has been shown previously that overexpression of Sec4p by pNB170 suppresses sec2-41 and sec2-59 at 34°C (Nair et al., 1990). We found that the bud tip staining of Sec4p at the restrictive temperature is restored in all three sec2 mutants upon overexpression of Sec4p (data not shown).
Discussion
SEC2 was previously shown to be essential for cell growth and vesicular Golgi–to–plasma membrane transport (Novick et al., 1980; Nair et al., 1990). In this paper, we establish a role for Sec2p in the polarized delivery of vesicles to sites of exocytosis. Several lines of evidence support a model in which Sec2p performs this function through its action as a Sec4p nucleotide exchange protein.
First, Sec4p is mislocalized in sec2 mutants as a result of an inefficient concentration of vesicles carrying Sec4p at the bud tip. Second, Sec2p interacts directly with Sec4p, preferentially in its nucleotide-free form, and through this interaction, it catalyzes nucleotide exchange on Sec4p. Third, although Sec2p is mostly cytosolic, a small pool of the protein cofractionates with secretory vesicles in a Sec4p-dependent fashion. We propose therefore that Sec2p cooperates with Sec4p in a process that leads to the polarized accumulation of secretory vesicles.
The targeting of carrier vesicles to their site of fusion is mediated by a complex machinery of proteins. It remains a central question which factors contribute to the specificity of vesicle targeting. Rab proteins are found to specifically localize to compartments involved in a particular step of membrane traffic (see Pfeffer, 1996; for review see Ferro-Novick and Novick, 1993). However, experiments in yeast using a chimeric protein that can function as both Sec4p and Ypt1p have excluded that Rab proteins are the sole determinants of vesicle targeting specificity (Brennwald and Novick, 1993). The SNARE hypothesis put forward by Rothman and colleagues proposes that the interaction of integral membrane proteins residing in the vesicle and target membranes forms the basis for correct targeting of carrier vesicles. According to this hypothesis, the interaction of specific pairs of vesicle SNAP receptors (v-SNAREs) and target SNAP receptors (t-SNAREs) allows for vesicle docking to the proper target membrane (Rothman and Warren, 1994). One proposal is that the interaction of v-SNAREs with t-SNAREs could be regulated by Rab proteins (Novick and Brennwald, 1993). In support of such a model, it has been shown that Sec9p, the yeast homologue of the mammalian t-SNARE SNAP-25, acts downstream of Sec4p (Brennwald et al., 1994). Our finding that Sec4p is correctly localized to the bud tip in sec9-4 mutant cells is in agreement with these data. So far, no evidence for a direct interaction between rab proteins and the v-SNARE/ t-SNARE complex has been found (Söllner et al., 1993; Lian et al., 1994; Brennwald et al., 1994; Søgaard et al., 1994). It is an open question as to which proteins could provide a link between Rab protein function and v-SNARE/ t-SNARE interaction.
Evidence is accumulating that the products of several late-acting SEC genes could provide such a link. Sec3p, Sec5p, Sec6p, Sec8p, Sec15p, and Exo70p, a newly identified protein, are all part of a multiprotein complex (Bowser and Novick, 1991; Bowser et al., 1992; TerBush et al., 1996). Sec8p, one component of this complex, now named the Exocyst, localizes to the bud tip and is therefore a good candidate for a downstream effector of Sec4p, possibly mediating vesicle docking (TerBush et al., 1995; Pfeffer, 1996). We have now shown that temperature-sensitive mutations in six of the genes that encode Exocyst components do not affect Sec4p localization. As demonstrated for sec6-4, vesicles carrying Sec4p are concentrated normally at the bud tip in these cells after a shift to the restrictive temperature. Although the Exocyst could nevertheless provide a link between Sec4p and the v-SNARE/t-SNARE complex, additional upstream factors are needed to account for the polarized transport and correct targeting of secretory vesicles. For several reasons, Sec2p is a good candidate for such a factor. First, vesicles carrying Sec4p require functional Sec2p to concentrate at the site of exocytosis. Sec2p is therefore required either to transport vesicles in a polarized fashion or to retain them at the site of exocytosis. Second, we found that Sec2p acts upstream of Sec6p since a sec2-78,6-4 double mutant shows the same phenotype of Sec4p immunofluorescence as the sec2-78 mutant alone. Thus, Sec2p probably acts before the Exocyst complex. This conclusion is supported by previous findings concerning Sec15p, another component of the exocyst. Sec15p has been proposed to function in docking since its overproduction results in the formation of a cluster of secretory vesicles (Salminen and Novick, 1989). This cluster appears by immunofluorescence as a bright spot of Sec15p labeling. In a sec4-8 or sec2-41 mutant background, but not in other late-acting sec mutants, the patch of Sec15p immunofluorescence is no longer observed. It has been therefore proposed that Sec4p and Sec2p might act upstream of Sec15p (Salminen and Novick, 1989). Interestingly, Sec2p itself seems to be part of a large protein complex that displays a native molecular mass of >500 kD (Nair et al., 1990). The concept emerging from these studies is that proper targeting and docking of secretory vesicles is not a function of one protein, but rather a multistep process that involves Sec4p, Sec4p-interacting proteins such as Sec2p, and a complex downstream machinery that includes the Exocyst and SNAREs. In support of the generality of such a model, Rab8 has been shown to promote the polarized delivery of vesicular stomatitis virus protein in transfected BHK cells (Peränen et al., 1996).
In mammalian cells, it has been recently demonstrated that Rab proteins bind to membranes initially as a Rab-GDP/GDI complex. Upon binding, GDI is released and only after a kinetic lag is conversion into the GTP form of the Rab proteins observed (Soldati et al., 1994; Ullrich et al., 1994). We show here that Sec2p fractionates with secretory vesicles in a Sec4p-dependent fashion, and its interaction with Sec4p catalyzes nucleotide exchange. We conclude that Sec2p acts after initial membrane binding of Sec4p and plays a role in the activation process. In support of this, we demonstrate that in sec2 mutants, Sec4p still localizes to secretory vesicles.
Another factor that stimulates guanine nucleotide release from Sec4p, Dss4p (dominant suppressor of Sec4p), has been described (Moya et al., 1993). By comparison to Sec2p, however, Dss4p is much less active in promoting GDP dissociation and, unlike Sec2p, cannot stimulate the association of GTP using comparable conditions (not shown). A further distinction is that while SEC2 is an essential gene, DSS4 can be deleted with little effect on growth or secretion. dss4Δ shows a synthetic negative interaction with sec2-41; however, overexpression of Dss4p does not suppress the growth defect of sec2-78, sec2-41, or sec2-59 (data not shown). It is unlikely, therefore, that Sec2p is functionally redundant with Dss4p; rather, we believe that they function cooperatively.
The direct interaction between Sec2p and Sec4p requires only the NH2-terminal half of Sec2p. This portion of Sec2p contains a predicted coiled-coil domain (Nair et al., 1990). The COOH-terminal domain of Sec2p is dispensable at the permissive temperature as the conditional lethal alleles sec2-41 and sec2-59 express a truncated version of Sec2p that lacks this portion (Nair et al., 1990). These data imply that the NH2-terminal portion of Sec2p contains the essential portion for its interaction with Sec4p and for its function in secretion.
Several hydrophilic proteins have been described that can interact with Rabs on transport vesicles (Kurzchalia et al., 1992; Shirataki et al., 1993; Li et al., 1994; Stenmark et al., 1995). Rabaptin5 is recruited to early endosomes by its interaction with Rab5-GTP. In addition, Rabaptin5 is required for early endosome fusion and is therefore a Rab5 effector (Stenmark et al., 1995). Rabphilin3A is another protein with specificity for an activated Rab, in this case Rab3A, although Rabphilin3A shares no homology with Rabaptin5 (Shirataki et al., 1993; Li et al., 1994; Stahl et al., 1996). Sec2p represents a different type of Rab-interacting protein whose affinity for the nucleotide-free form of Sec4p is consistent with its role as an activator rather than an effector. A GEF specific for Rab3A has been reported recently; this protein shares no sequence similarity to Sec2p, and unlike Sec2p, it requires a prenylated substrate (Wada et al., 1997). Sec2p shares sequence homology with Rabin3, a protein interacting with Rab3A/3D (Brondyk et al., 1995) and other novel EST sequences. The role of Rabin in Rab3-mediated secretion is not known, although it is interesting to note that Rabin shows allele-specific interactions to Rab3A (Brondyk et al., 1995). We suggest that these Sec2-like proteins may function as Rab activators in diverse eukaryotic organisms.
How does the activation of Sec4p by Sec2p contribute to the polarized concentration of vesicles at the bud tip? There are several possibilities. Vesicular traffic to the bud tip may require previous activation of Sec4p on the vesicle membrane by Sec2p. In this model, Sec4-GTP would serve as a trigger for polarized transport or for vesicle retention at sites of active exocytosis. Alternatively, Sec2p could directly mediate vesicle transport. In this case, Sec2p would bind to Sec4p and serve as a connection to the transport machinery, perhaps involving the cytoskeleton. Only after vesicles arrive at the bud tip would Sec2p dissociate and Sec4p adopt its GTP-bound conformation. This is an interesting possibility, since we have found that in act1-1 and myo2-66, Sec4p is randomly localized, as in sec2 mutants. Finally, Sec2p might only interact with Sec4p on vesicles after they arrive in the bud, where activation of Sec4p would lead to vesicle docking. Future experiments will further elucidate the mechanisms involved.
Acknowledgments
This work was supported by grants to P. Novick from the National Institutes of Health (GM35370 and CA46128) and from the Patrick and Catherine Weldon Donaghue Foundation, by a fellowship of the Deutsche Forschungsgemeinschaft to C. Walch-Solimena, and a long-term fellowship to R.N. Collins from the Human Frontier Science Program.
Footnotes
1. Abbreviations used in this paper: EST, expressed sequence tag; GEF, guanine nucleotide exchange factor; MBP, maltose binding protein; t-SNARE, target SNAP receptor; v-SNARE, vesicle SNAP receptor.
We are deeply indebted to Dr. B. Govindan for help with electron microscopy experiments, her interest in the project, and helpful discussions. We thank Dr. K.A. Joiner and S. Kupfer for providing constructs, L. Chicone and L. Hartnell for help and advice in electron microscopy techniques, Dr. C.W. Slayman for the plasma membrane ATPase antibody, Dr. G. Whittaker for experimental advice, and Dr. M. Solimena for critical reading of the manuscript.
Christiane Walch-Solimena and Ruth Collins contributed equally to this paper.
Please address all correspondence to Peter J. Novick, Department of Cell Biology, Yale University School of Medicine, New Haven, CT 06510. Tel.: (203) 785-5871; Fax: (203) 785-7226; E-mail: peter_novick@quickmail.cis.yale.edu
References
- Aalto MK, Ronne H, Keränen S. Yeast syntaxins Sso1p and Sso2p belong to a family of related membrane proteins that function in vesicular transport. EMBO (Eur Mol Biol Organ) J. 1993;12:4095–4104. doi: 10.1002/j.1460-2075.1993.tb06093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Araki S, Kaibuchi K, Sasaki T, Hata Y, Takai Y. Role of the C-terminal region of smg p25A in its interaction with membranes and the GDP/GTP exchange protein. Mol Cell Biol. 1991;11:1438–1447. doi: 10.1128/mcb.11.3.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature (Lond) 1990;348:125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Bowser R, Novick P. Sec15 protein, an essential component of the exocytotic apparatus, is associated with the plasma membrane and with a soluble 19.5 S particle. J Cell Biol. 1991;112:1117–1131. doi: 10.1083/jcb.112.6.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser R, Müller H, Govindan B, Novick P. Sec8p and Sec15p are components of a plasma membrane-associated 19.5 S particle. J Cell Biol. 1992;118:1041–1056. doi: 10.1083/jcb.118.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennwald P, Novick P. Interactions of three domains distinguishing the Ras-related GTP-binding proteins Ypt1 and Sec4. Nature (Lond) 1993;362:560–563. doi: 10.1038/362560a0. [DOI] [PubMed] [Google Scholar]
- Brennwald P, Kearns B, Champion K, Keranen S, Bankaitis V, Novick P. Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell. 1994;79:245–258. doi: 10.1016/0092-8674(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Brondyk WH, McKiernan CJ, Fortner KA, Stabila P, Holz RW, Macara IG. Interaction cloning of Rabin3, a novel protein that associates with the Ras-like GTPase Rab3A. Mol Cell Biol. 1995;15:1137–1143. doi: 10.1128/mcb.15.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien CT, Bartel PL, Sternglanz R, Fields S. The two-hybrid system: a method to identify and clone genes for proteins that interact with a protein of interest. Proc Natl Acad Sci USA. 1991;88:9578–9582. doi: 10.1073/pnas.88.21.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, R.N., P. Brennwald, M. Garrett, A. Lauring, and P. Novick. 1997. Interactions of nucleotide release factor DSS4 with SEC4 in the post-golgi secretory pathway of yeast. J. Biol. Chem. In press. [DOI] [PubMed]
- Drubin DG, James W, Nelson Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- Farnsworth CL, Feig LA. Dominant inhibitory mutations in the Mg(2+)-binding site of RasH prevent its activation by GTP. Mol Cell Biol. 1991;11:4822–4829. doi: 10.1128/mcb.11.10.4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro-Novick S, Novick P. The role of GTP-binding proteins in transport along the exocytic pathway. Ann Rev Cell Biol. 1993;9:575–599. doi: 10.1146/annurev.cb.09.110193.003043. [DOI] [PubMed] [Google Scholar]
- Garrett MD, Zahner JE, Cheney CM, Novick PJ. GDI1encodes a GDP dissociation inhibitor that plays an essential role in the yeast secretory pathway. EMBO (Eur Mol Biol Organ) J. 1994;13:1718–1722. doi: 10.1002/j.1460-2075.1994.tb06436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz RD, Schiestl RH. Applications of high efficiency lithium acetate transformation of intact yeast cells using single-stranded nucleic acids as carrier. Yeast. 1991;7:253–263. doi: 10.1002/yea.320070307. [DOI] [PubMed] [Google Scholar]
- Goud B, Salminen A, Walworth NC, Novick PJ. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 1988;53:753–768. doi: 10.1016/0092-8674(88)90093-1. [DOI] [PubMed] [Google Scholar]
- Govindan B, Novick P. Development of cell polarity in budding yeast. J Exp Zool. 1995;273:401–424. doi: 10.1002/jez.1402730505. [DOI] [PubMed] [Google Scholar]
- Govindan B, Bowser R, Novick P. The role of Myo2, a yeast class V myosin, in vesicular transport. J Cell Biol. 1995;128:1055–1068. doi: 10.1083/jcb.128.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoueix-Lerosey I, Jollivet F, Camonis J, Marche PN, Goud B. Two-hybrid system screen with the small GTP-binding protein Rab6. Identification of a novel mouse GDP dissociation inhibitor isoform and two other potential partners of Rab6. J Biol Chem. 1995;270:14801–14808. doi: 10.1074/jbc.270.24.14801. [DOI] [PubMed] [Google Scholar]
- Johnston GC, Prendergast JA, Singer RA. The Saccharomyces cerevisiae MYO2gene encodes an essential myosin for vectorial transport of vesicles. J Cell Biol. 1991;113:539–551. doi: 10.1083/jcb.113.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabcenell AK, Goud B, Northup JK, Novick P. Binding and hydrolysis of guanine nucleotides by Sec4p, a yeast protein involved in the regulation of vesicular traffic. J Biol Chem. 1990;265:9366–9372. [PubMed] [Google Scholar]
- Kaiser CA, Schekman R. Distinct sets of SECgenes govern transport vesicle formation and fusion early in the secretory pathway. Cell. 1990;61:723–733. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- Kurzchalia TV, Gorvel JP, Dupree P, Parton R, Kellner R, Houthaeve T, Gruenberg J, Simons K. Interactions of Rab5 with cytosolic proteins. J Biol Chem. 1992;267:18419–18423. [PubMed] [Google Scholar]
- Li C, Takei K, Geppert M, Daniell L, Stenius K, Chapman ER, Jahn R, De Camilli P, Südhof TC. Synaptic targeting of rabphilin-3A, a synaptic vesicle Ca2+/phospholipid-binding protein, depends on rab3A/3C. Neuron. 1994;13:885–898. doi: 10.1016/0896-6273(94)90254-2. [DOI] [PubMed] [Google Scholar]
- Lian JP, Ferro-Novick S. Bos1p, an integral membrane protein of the endoplasmic reticulum to Golgi transport vesicles, is required for their fusion competence. Cell. 1993;73:735–745. doi: 10.1016/0092-8674(93)90253-m. [DOI] [PubMed] [Google Scholar]
- Lian JP, Stone S, Jiang Y, Lyons P, Ferro-Novick S. Ypt1p implicated in v-SNARE activation. Nature (Lond) 1994;372:698–701. doi: 10.1038/372698a0. [DOI] [PubMed] [Google Scholar]
- Moya M, Roberts D, Novick P. DSS4-1 is a dominant suppressor of sec4-8that encodes a nucleotide exchange protein that aids Sec4p function. Nature (Lond) 1993;361:460–463. doi: 10.1038/361460a0. [DOI] [PubMed] [Google Scholar]
- Mulholland A, Preuss D, Moon A, Wong A, Drubin D, Botstein D. Ultrastructure of the yeast actin cytoskeleton and its association with the plasma membrane. J Cell Biol. 1994;125:381–391. doi: 10.1083/jcb.125.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair J, Müller H, Peterson M, Novick P. Sec2 protein contains a coiled-coil domain essential for vesicular transport and a dispensable carboxy terminal domain. J Cell Biol. 1990;110:1897–1909. doi: 10.1083/jcb.110.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick PJ, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Novick P, Botstein D. Phenotypic analysis of temperature-sensitive yeast actin mutants. Cell. 1985;40:405–416. doi: 10.1016/0092-8674(85)90154-0. [DOI] [PubMed] [Google Scholar]
- Novick P, Brennwald P. Friends and family: the role of the Rab GTPases in vesicular traffic. Cell. 1993;75:597–601. doi: 10.1016/0092-8674(93)90478-9. [DOI] [PubMed] [Google Scholar]
- Novick PJ, Goud B, Salminen A, Walworth NC, Nair J, Potenza M. Regulation of vesicular traffic by a GTP-binding protein on the cytoplasmic surface of secretory vesicles in yeast. Cold Spring Harb Symp Quant Biol. 1988;53:637–467. doi: 10.1101/sqb.1988.053.01.073. [DOI] [PubMed] [Google Scholar]
- Peränen J, Auvinen P, Virta H, Wepf R, Simons K. Rab8 promotes polarized membrane transport through reorganization af actin and microtubules in fibroblasts. J Cell Biol. 1996;135:153–167. doi: 10.1083/jcb.135.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR. Transport vesicle docking—SNAREs and associates. Ann Rev Cell Devel Biol. 1996;12:441–461. doi: 10.1146/annurev.cellbio.12.1.441. [DOI] [PubMed] [Google Scholar]
- Preuss D, Mulholland J, Franzusoff A, Segev N, Botstein D. Characterization of the SaccharomycesGolgi complex through the cell cycle by immunoelectron microscopy. Mol Cell Biol. 1992;3:789–803. doi: 10.1091/mbc.3.7.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopov V, Govindan B, Novick P, Gerst JE. Homologs of the synaptobrevin/VAMP family of synaptic vesicle proteins function on the late secretory pathway in S. cerevisiae. . Cell. 1993;74:855–861. doi: 10.1016/0092-8674(93)90465-3. [DOI] [PubMed] [Google Scholar]
- Roberts DM, Garrett MD, Novick P. Purification of the GDP dissociation stimulator Dss4 from recombinant bacteria. Methods Enzymol. 1995;257:84–92. doi: 10.1016/s0076-6879(95)57013-6. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Warren G. Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr Biol. 1994;4:220–233. doi: 10.1016/s0960-9822(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., E.F. Fritsch, and T. Maniatis. 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Salminen A, Novick PJ. The Sec15 protein responds to the function of the GTP binding protein, Sec4, to control vesicular traffic in yeast. J Cell Biol. 1989;109:1023–1036. doi: 10.1083/jcb.109.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirataki H, Kaibuchi K, Sakoda T, Kishida S, Yamaguchi T, Wada K, Miyazaki M, Takai Y. Rabphilin-3A, a putative target protein for smg p25A/rab3A p25 small GTP-binding protein related to synaptotagmin. Mol Cell Biol. 1993;13:2061–2068. doi: 10.1128/mcb.13.4.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon I, Zerial M, Goody RS. Kinetics of interaction of Rab5 and Rab7 with nucleotides and magnesium ions. J Biol Chem. 1996;271:20470–20478. doi: 10.1074/jbc.271.34.20470. [DOI] [PubMed] [Google Scholar]
- Søgaard M, Tani K, Ye RR, Geromanos S, Tempst P, Kirchhausen T, Rothman JE, Söllner T. A Rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell. 1994;78:937–948. doi: 10.1016/0092-8674(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Soldati T, Shapiro AD, Svejstrup AB, Pfeffer SR. Membrane targeting of the small GTPase Rab9 is accompanied by nucleotide exchange. Nature (Lond) 1994;369:76–78. doi: 10.1038/369076a0. [DOI] [PubMed] [Google Scholar]
- Söllner T, Bennett M, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation and fusion. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Stahl B, Chou JH, Li C, Südhof TC, Jahn R. Rab3 reversibly recruits rabphilin to synaptic vesicles by a mechanism analogous to raf recruitment by ras. EMBO (Eur Mol Biol Organ) J. 1996;15:1799–1809. [PMC free article] [PubMed] [Google Scholar]
- Stenmark H, Parton RG, Steele-Mortimer O, Lutcke A, Gruenberg J, Zerial M. Inhibition of Rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO (Eur Mol Biol Organ) J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H, Vitale G, Ullrich O, Zerial M. Rabaptin5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell. 1995;83:423–432. doi: 10.1016/0092-8674(95)90120-5. [DOI] [PubMed] [Google Scholar]
- TerBush DR, Novick P. Sec6, Sec8, and Sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae. . J Cell Biol. 1995;130:299–312. doi: 10.1083/jcb.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush DR, Maurice T, Roth D, Novick P. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. . EMBO (Eur Mol Biol Organ) J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- Ullrich O, Horiuchi H, Bucci C, Zerial M. Membrane association of Rab5 mediated by GDP-dissociation inhibitor and accompanied by GDP/ GTP exchange. Nature (Lond) 1994;368:157–160. doi: 10.1038/368157a0. [DOI] [PubMed] [Google Scholar]
- Wada M, Nakanishi H, Satoh A, Hirano H, Obaishi H, Matsuura Y, Takai Y. Isolation and characterization of a GDP/GTP exchange protein specific for the Rab3 subfamily of small G proteins. J Biol Chem. 1997;272:3875–3878. doi: 10.1074/jbc.272.7.3875. [DOI] [PubMed] [Google Scholar]
- Walworth NC, Novick PJ. Purification and characterization of constitutive secretory vesicles from yeast. J Cell Biol. 1987;105:163–174. doi: 10.1083/jcb.105.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walworth NC, Goud B, Kabcenell AK, Novick PJ. Mutational analysis of SEC4suggests a cyclical mechanism for the regulation of vesicular traffic. EMBO (Eur Mol Biol Organ) J. 1989;8:1685–1693. doi: 10.1002/j.1460-2075.1989.tb03560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walworth NC, Brennwald P, Kabcenell AK, Garrett M, Novick P. Hydrolysis of GTP by Sec4 protein plays an important role in vesicular transport and is stimulated by a GTPase-activating protein in Saccharomyces cerevisiae. . Mol Cell Biol. 1992;12:2017–2028. doi: 10.1128/mcb.12.5.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Schreiber SL. Cloning, Zn2+binding, and structural characterization of the guanine nucleotide exchange factor human Mss4. Biochemistry. 1995;34:9103–9110. doi: 10.1021/bi00028a020. [DOI] [PubMed] [Google Scholar]
- Zerial M, Stenmark H. Rab GTPases in vesicular transport. Curr Opin Cell Biol. 1993;5:613–620. doi: 10.1016/0955-0674(93)90130-i. [DOI] [PubMed] [Google Scholar]