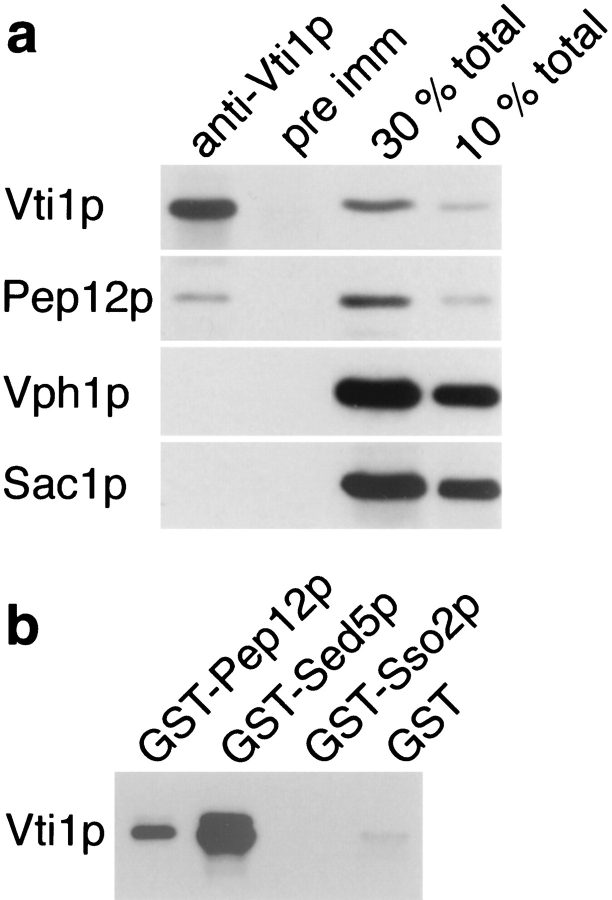
Figure 9.
Physical interaction of Vti1p with Sed5p and Pep12p. (a) Coimmunoprecipitation of Pep12p with Vti1p. Yeast spheroplasts were solubilized with 0.5% Triton X-100. Vti1p was quantitatively precipitated from the Triton extract by native immunoprecipitation with anti-Vti1p antibodies, and ∼10% of the total Pep12p was coimmunoprecipitated. In contrast, the membrane proteins Vph1p (vacuole) and Sac1p (ER and Golgi apparatus) were not coimmunoprecipitated, and pre-immune serum did not precipitate any of these proteins. For quantitative comparison, Triton extracts equivalent to 30 and 10% of total protein present in the immunoprecipitations were analyzed in lanes 3 and 4. The proteins bound to the beads and the starting extracts were analyzed by SDS-PAGE and immunoblotting with antibodies against Vti1p, Pep12p, Vph1p, and Sac1p. (b) In vitro binding of the soluble domain of Vti1p to immobilized GST fusion proteins containing the soluble domains of different yeast t-SNAREs. Vti1p interacted with GST-Sed5p and GST-Pep12p but not with GST-Sso2p or GST alone. Equal amounts of immobilized GST fusion proteins were incubated with 6-His–Vti1p. The bound proteins were analyzed by SDS-PAGE and immunoblotting with anti-Vti1p antibodies.