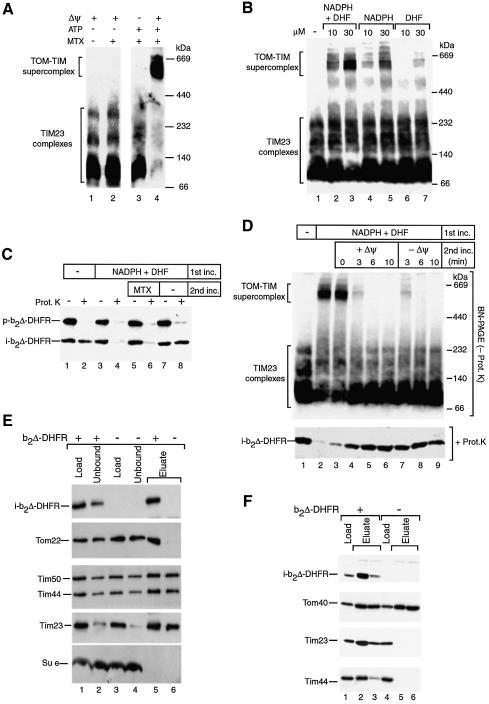
Fig. 1. The TOM–TIM supercomplex represents a productive translocation intermediate. (A) Formation of the supercomplex. Purified b2Δ-DHFR was incubated with wild-type yeast mitochondria for 15 min at 25°C in the presence or absence of MTX. For sample 3, Δψ was dissipated before the import reaction. For samples 1 and 2, the import assay was depleted of ATP. After the import reaction, the mitochondria were reisolated, lyzed with digitonin and subjected to BN–PAGE. Analysis was performed by immunodecoration with Tim23-specific antibodies. (B) Generation of the supercomplex in the presence of NADPH and DHF. b2Δ-DHFR was imported into isolated mitochondria for 12 min at 25°C, followed by BN–PAGE and immunodecoration for Tim23. (C) Two-step import assay. Purified b2Δ-DHFR was incubated with mitochondria for 15 min at 25°C in the presence or absence of 30 µM NADPH and 30 µM DHF (first incubation). Upon reisolation, the mitochondria of samples 5–8 were resuspended in fresh import buffer and incubated for a further 10 min at 25°C (second incubation). Where indicated, the mitochondria were treated with proteinase K. The samples were separated by SDS–PAGE and immunodecorated with DHFR-specific antibodies. p, precursor; i, processed (intermediate-sized) form of b2Δ-DHFR. (D) Purified b2Δ-DHFR was arrested in mitochondria in the presence or absence of 30 µM NADPH and 30 µM DHF. Upon reisolation, the mitochondria of samples 3–9 were resuspended in fresh import buffer and incubated for the indicated times at 25°C in the presence or absence of a Δψ as indicated. The samples were divided in half. One portion was subjected to BN–PAGE and decorated with Tim23-specific antibodies (upper panel). The other portion was treated with proteinase K, separated by SDS–PAGE and decorated with DHFR-specific antibodies (lower panel). (E) Affinity-purification of the supercomplex via Tim23ProtA. Where indicated, b2Δ-DHFR was accumulated in yeast mitochondria carrying Tim23ProtA in the presence of MTX. The mitochondria were reisolated, lyzed with digitonin, and subjected to IgG affinity chromatography, SDS–PAGE and immunodecoration. Thirty percent of the load and unbound material and 100% of the eluate are shown. (F) Affinity-purification of the supercomplex via Tom22His10. Where indicated, b2Δ-DHFR was accumulated in mitochondria carrying Tom22His10 in the presence of MTX. The reisolated mitochondria were lyzed with digitonin, and subjected to Ni-NTA affinity chromatography, SDS–PAGE and immunodecoration. Ten percent of load and 100% of the eluate fractions are shown.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.