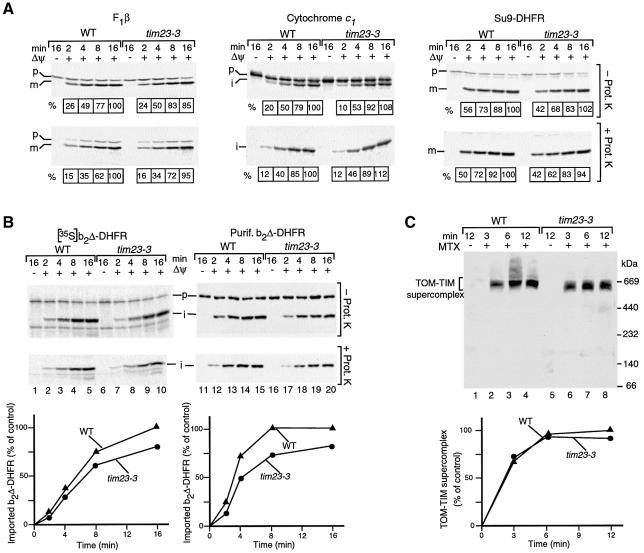
Fig. 3. A connection of mitochondrial outer and inner membranes via Tim23 is not critical for formation of the TOM–TIM supercomplex. (A) Import of preproteins into tim23-3 mitochondria. The 35S-labeled precursors of F1-ATPase subunit β (F1β), cytochrome c1 and Su9-DHFR were incubated with wild-type (WT) or tim23-3 mitochondria at 25°C. Where indicated, the mitochondria were treated with proteinase K after the import reaction. The mitochondria were reisolated and analyzed by SDS–PAGE and digital autoradiography. The amount of processed protein in energized wild-type mitochondria after an import time of 16 min was set to 100%, respectively. p, precursor; i, m, processed forms (intermediate-sized, mature) of the protein. (B) 35S-labeled precursor of b2Δ-DHFR (left panel) or purified b2Δ-DHFR (right panel) were imported into wild-type or tim23-3 mitochondria. The amount of protease-protected, processed i-b2Δ-DHFR in energized wild-type mitochondria after an import time of 16 min was set to 100% (control). (C) Formation of the TOM–TIM supercomplex. Purified b2Δ-DHFR was incubated with wild-type or tim23-3 mitochondria in the presence of MTX unless indicated otherwise. The mitochondria were reisolated, lyzed with digitonin and subjected to BN–PAGE and immunodecoration with antibodies directed against DHFR. The amount of supercomplex formed after an incubation of 12 min in wild-type mitochondria was set to 100% (control).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.