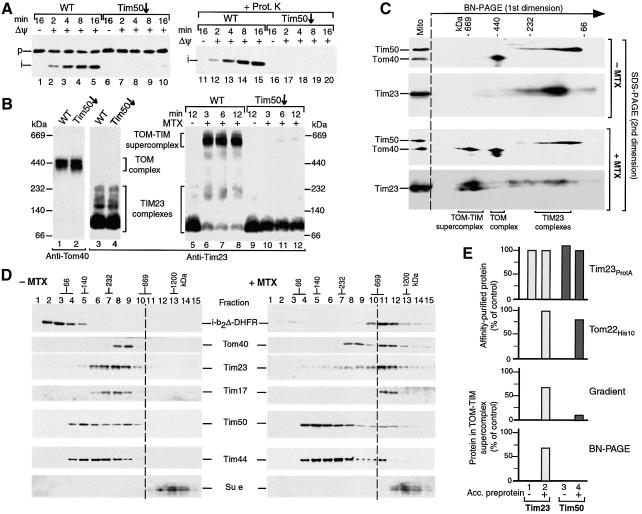
Fig. 6. The TOM–TIM supercomplex is not dissociated by a release of Tim50. (A) Import of b2Δ-DHFR is inhibited by depletion of Tim50. Purified b2Δ-DHFR was imported into Tim50-depleted or wild-type (WT) mitochondria. The reisolated mitochondria were separated by SDS–PAGE, followed by immunodecoration with antibodies against DHFR. (B) Generation of the supercomplex is blocked in Tim50-depleted mitochondria. Samples 1–4, isolated wild-type mitochondria and Tim50-depleted mitochondria (70 µg protein each) were lyzed with digitonin and subjected to BN–PAGE and immunodecoration as indicated (absence of preprotein). Samples 5–12, purified b2Δ-DHFR was arrested in wild-type or Tim50-depleted mitochondria (70 µg protein) in the presence of MTX, unless indicated otherwise. The mitochondria were reisolated and subjected to BN–PAGE and immunodecoration with Tim23-specific antibodies. (C) The supercomplex separated by BN–PAGE does not contain Tim50. Purified b2Δ-DHFR was imported into wild-type mitochondria in the presence or absence of MTX for 15 min at 25°C. Protein complexes were separated by 2D PAGE (BN–PAGE followed by SDS–PAGE). Tim50, Tom40 and Tim23 were detected by immunodecoration. (D) Sucrose gradient analysis of the supercomplex. Purified b2Δ-DHFR was imported into wild-type mitochondria in the presence or absence of MTX. The mitochondria were reisolated and lyzed with digitonin. Protein complexes were separated by sucrose gradient centrifugation. Fractions were collected, separated by SDS–PAGE and individual proteins were detected by immunodecoration (15 out of 20 fractions are shown). (E) Quantitative assessment of the presence of Tim50 in the supercomplex. Where indicated, b2Δ-DHFR was accumulated (acc.) in energized mitochondria in the presence of MTX. Top panel, Tim23ProtA mitochondria; second panel, Tom22His10 mitochondria; third and fourth panels, wild-type mitochondria. The mitochondria were reisolated and lyzed with digitonin and subjected to either IgG-affinity chromatography (top panel), Ni-NTA affinity chromatography (second panel), sucrose gradient centrifugation (third panel) or BN–PAGE (fourth panel). The amounts of Tim23 and Tim50 were determined by immunodecoration. First and second panels, the recovery of affinity- purified Tim23 from mitochondria with accumulated b2Δ-DHFR was set to 100% (control). Third and fourth panels, the total amount of solubilized Tim23 and Tim50 was set to 100% (control), respectively.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.