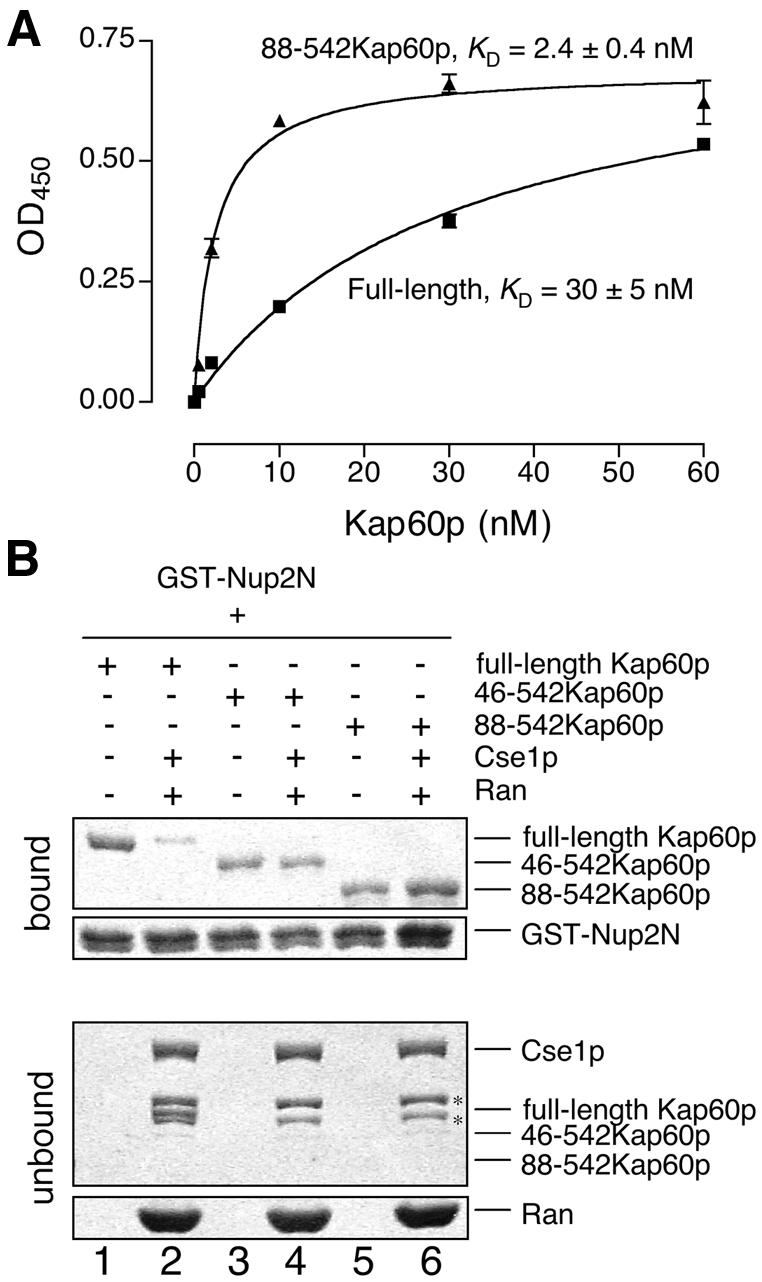
Fig. 6. Competition with the IBB domain and Cse1p/RanGTP. (A) Kap60p (filled squares) binds more weakly to Nup2N than Kap60Δ (filled triangles). Each data point was performed in duplicate and error bars represent SEM. (B) Cse1p:RanGTP competes Kap60p from Nup2N when the IBB domain is present. GST–Nup2N (10 µg) was treated with 3 µM Kap60p ± Cse1p (15 µg):Ran (30 µg) in binding buffer supplemented with 5 mM Mg(OAc)2. Two bands marked with * in the unbound fractions are copurifying bacterial proteins present in Cse1p. Full-length Kap60p can be seen between these two bands in lane 2.
