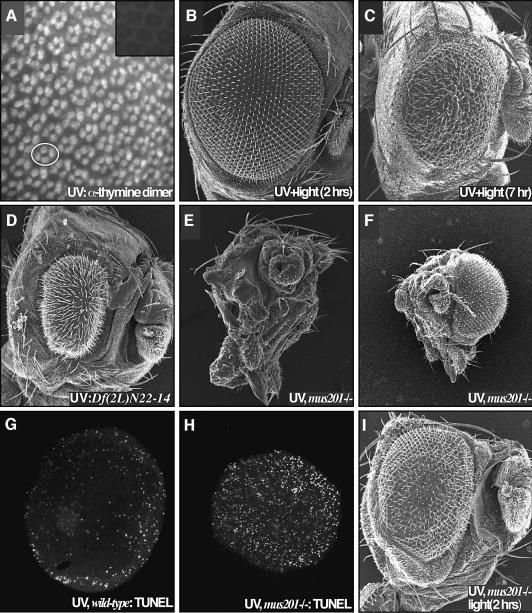
Fig. 2. Retinal defects following UV irradiation are due to DNA damage. (A) Irradiation of 24 h APF retinae resulted in a rapid induction of pyrimidine dimers. Each four-cell group is the cone cell nuclei of one ommatidium; an example is circled. The inset presents a non-irradiated control. (B) Exposure of the retina to light 2 h after irradiation resulted in a complete suppression of the UV retinal phenotype due to photoreactivation- mediated repair of the pyrimidine dimers. (C) Photoreactivating light failed to fully rescue the retina when applied 7 h after irradiation. (D) A deficiency removing the 29–30C region of the second chromosome strongly enhanced the UV phenotype (compare with Figure 1B). This region is predicted to contain xpg/mus201, along with a number of other loci. (E) Removing both functional copies of the nucleotide excision repair gene xpg/mus201, using the mus201D1 allele, resulted in a complete ablation of the retina following irradiation. The dying pupa was removed from its pupal case; few structures are identifiable as the entire retina and much of the head is affected. (F) A frontal view of this animal reveals the extent of the damage. The non-irradiated retina showed no defects. A comparison of TUNEL staining in wild type (G) and the xpg/mus201D1 mutant (H) revealed that the xpg/mus201 phenotype was due to a dramatic increase in apoptotic cell death (42 h APF). (I) Exposure of the xpg/mus201D1 retina to light following irradiation resulted in suppression of the lethality as well as retinal and head defects.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.