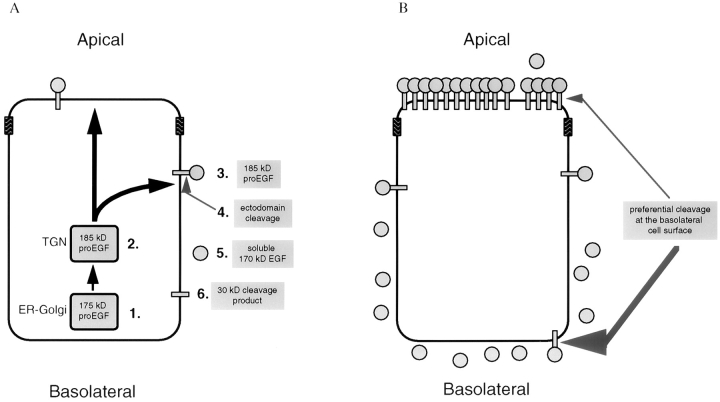
Figure 8.
A proposed model for the establishment and maintenance of an apical distribution of proEGF in polarized MDCK cells. (A) Biosynthesis and sorting of proEGF in MDCK cells. 1. 175-kD proEGF represents the endo H–sensitive, intracellular form of proEGF. 175-kD proEGF is modified during transit through the secretory pathway to form 185-kD proEGF. 2. Newly synthesized 185-kD proEGF is not sorted at the level of the TGN but is delivered equally to both apical and basolateral membrane domains. 3. 185-kD proEGF represents the endo H–resistant, cell surface form of proEGF. 4. Ectodomain cleavage of cell surface 185-kD proEGF. 5. 170-kD EGF represents a soluble high–molecular mass EGF obtained by ectodomain cleavage of cell surface 185-kD proEGF. 6. 30-kD cleavage product represents the cytoplasmic and transmembrane domains found after cell surface protease digestion. (B) In polarized MDCK cells, preferential ectodomain cleavage of cell surface proEGF at the basolateral membrane domain leads to an apical enrichment of proEGF. Enhanced basolateral processing also leads to an accumulation of soluble 170-kD EGF in the basolateral medium. A matrix metalloprotease inhibitor, batimastat, dramatically inhibits processing of 185-kD proEGF at the cell surface.