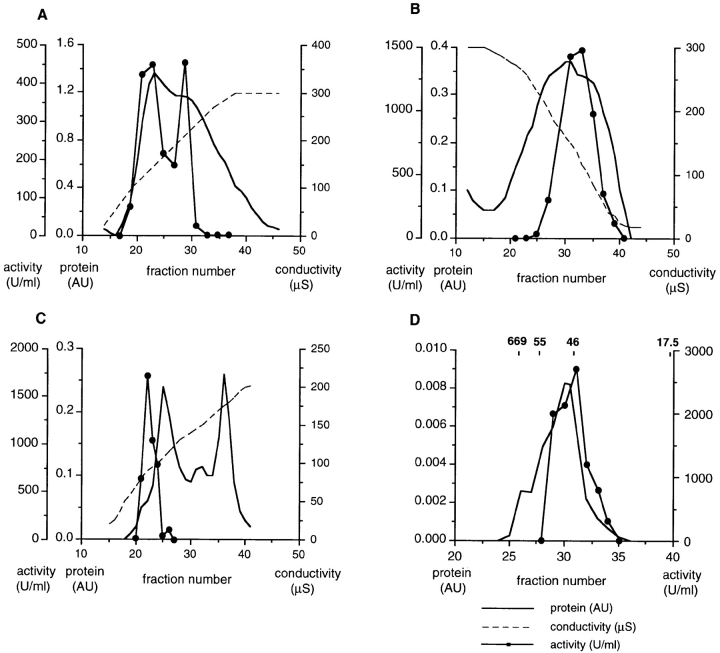
Figure 5.
Purification of the active component from pig brain cytosol. (A) Cibachrome blue 3GA: a step-eluted fraction from fast-flow Q-Sepharose, diluted to 50 mM salt, was passed over a 40 ml Cibachrome blue 3GA column, washed extensively, and eluted as shown in a continuous 25-300 μS salt gradient. Protein was monitored in line by absorbence at 280 nm, and salt concentration by conductivity, as shown. The activities of column fractions were assayed and plotted (far left ordinate axis) and the two activity peaks were pooled. (B) Phenyl–Sepharose: pooled fractions from A were applied to a 15-ml phenyl–Sepharose column and proteins eluted with a 300–25 μS salt gradient. (C) Q-Sepharose: pooled active fractions from B were diluted to 20 mM salt and chromatographed on a 5-ml Q-Sepharose column. Activity was eluted in a salt gradient to 200 μS. (D) 200 μl of the peak fraction from C was applied to a Bio-Silect gel filtration column with a rated separation range of 100–5 kD. The elution positions of molecular weight standards are indicated.