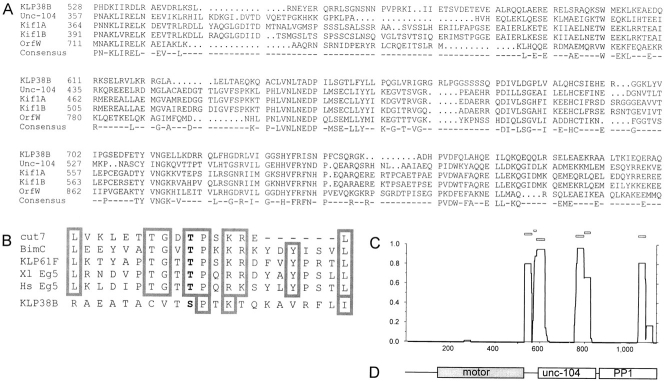
Figure 2.
Sequence alignments. (A) Unc104-related kinesin–related proteins share similarity outside the motor domain. Alignment of KLP38B residues 528–826 with similar kinesin-related proteins, using University of Wisconsin GCG program PILEUP. P528 is the last residue of the conserved region shared between all kinesin–related proteins, containing the motor domain. OrfW (D26361) is the predicted product of cDNA KIAA0042 from Homo sapiens (Nomura et al., 1994), Kif1A (D29951) and Kif1B (D17577) are from Mus musculus (Okada et al., 1995; Nangaku et al., 1994), and Unc-104 (M58582) is from Caenorhabditis elegans (Otsuka et al., 1991). (B) KLP38B potential cdk phosphorylation site is not related to the BimC/Eg5 subfamily. Comparison of the putative cdk phosphorylation site in KLP38B with that of BimC/Eg5-like kinesin-related proteins. Of the residues common to the BimC/Eg5 sequences, only the core cdk consensus (S/T P × K/R) and a leucine are also present in KLP38B. (C) Predicted coiled-coil formation by KLP38B. PEPCOIL output: probabilities of the KLP38B protein forming α-helical coiled-coils as predicted from the algorithms of Lupas et al. (1991), using a 28-residue window. The horizontal axis is position within the protein, and the NH2 terminus is at the origin; the vertical axis is probability of coiled-coil formation. Open boxes above the plot indicate the frame of the coiled-coil repeat. (D) Domain structure of KLP38B. The motor domain, region of extended sequence similarity with Unc-104–related KRPs, and the COOH-terminal PP1-binding region are shown at the same scale as in C. The PP1-binding region is defined by the shortest KLP38B clone from the two-hybrid screen.