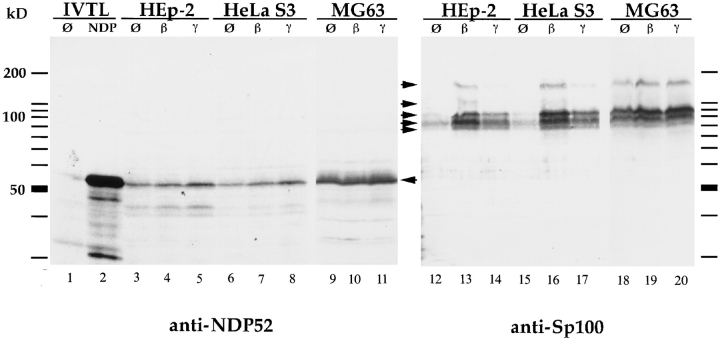
Figure 1.
Immunoblot analysis of in vitro–translated protein and total cell extracts of HEp-2, HeLa S3, and MG63 cells, separated by 12.5% SDS-PAGE. In vitro–translated NDP52 detected by the polyclonal rabbit anti-NDP52 antiserum exhibits an electrophoretic mobility corresponding to a protein of 56–58 kD (lane 2). No specific reaction is seen using reticulocyte lysate alone (lane 1). In HEp-2, HeLa S3, and MG63 cells, endogenous NDP52 has the same electrophoretic mobility (lanes 3–11, marked by an arrow). Treatment of cells with IFN β (lanes 4, 7, and 10) and γ (lanes 5, 8, and 11) does not increase the amount of NDP52 (IFN β), or only increases it marginally (IFN γ). Immunoblot analysis of the same extracts using a polyclonal rat anti-Sp100 antiserum (lanes 12–20) in the HEp-2 and HeLa S3 cell lines shows a strong induction of Sp100 proteins (specific complexes indicated by arrows) by IFN β (lanes 13 and 16) and a weaker induction by IFN γ (lanes 14 and 17). MG63 cells, in contrast, showed only weak inducibility by both IFN β (lane 19) and IFN γ (lane 20).