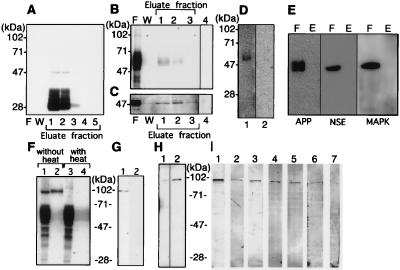
Figure 2.
Analysis of PS1-associated tau and GSK-3β in human brain. Triton X-100 soluble fractions (10 ml) of clinically normal human brain (2 g) was loaded onto a MKAD3.4 immuno-affinity column to purify PS1 and associated proteins. (A) Purification profiles show the flow-through fraction (20 μl) (F), the fraction of last wash (20 μl/total 5 ml) by TBS containing 1% Triton X-100 (W), and purified fraction (20 μl per lane was loaded from total 500 μl eluate) (Eluate). The immuno-detection of each fraction by using MKAD3.4 (1:5) did not show any reactivity in the flow-through or the wash. Eluate fractions 1 and 2 revealed abundant reactivity corresponding to the 47-kDa full length and 28-kDa N-terminal fragment of PS1. JM (1:3,000) was used for immuno-detection of tau (B), and anti-GSK-3β (1:1,000) was used to detect GSK-3β (Transduction Laboratories) (C). Most of the tau and GSK-3β was recovered in the flow through fractions (20 μl). The JM antibody reacted with bands at 50–70 kDa, whereas anti-GSK-3β reacted with a band at 47 kDa. Reactivity for both antibodies was recovered in fractions 1 and 2 (20 μl). Only secondary antibody incubation, as negative control, did not show any visible bands in the eluate fraction 1 (20 μl) (lane 4). (D) The JM reactive band in eluate fraction 1 (20 μl) (lane 1) was eliminated by preabsorption with human recombinant tau (lane 2). No reactivity is seen in lane 2. (E) Eluate fraction 1 (lane E) and flow-through fraction (lane F) were incubated with anti-NSE, anti-APP, and anti-MAPK. No reactivities are seen in eluate fraction. (F) The 95-kDa band in the eluate from fraction 1 (20 μl) could be eliminated by heating before loading on the gel (lane 1, lysate without heating; lane 2, eluate without heating; lane 3, lysate with heating; lane 4, lysate without heating). The heating process appeared to convert the 95-kDa JM-reactive band into a set of JM-reactive bands in 50- to 70-kDa region, which is characteristic of tau. (G) The 95-kDa band (lane 1) in eluate fraction 1 was absorbed away by preabsorption of JM with human recombinant tau (lane 2). (H) The 95-kDa band was weakly reactive with a nonphosphorylation-dependent anti-tau antibody (tau-1; 1:5,000), (20 μl of a 5-fold concentrated eluate was used) (lane 1). Alkaline phosphatase (130 units/ml, Takara Shuzo, Kyoto) treatment of the same sample for 1 hr at 4°C enhanced the immunoreactivity of tau-1 (lane 2). (I) The phosphorylation sites present in the 95-kDa band was determined by using well characterized phosphorylation-dependent anti-tau antibodies (33, 35). The phosphorylation sites on tau (in the numbering of htau40) identified by each antibody correspond to: lane 1, PS199 (1:200), Ser-199; lane 2, PS202 (1:200), Ser-202; lane 3, PS262 (1:200), Ser-262; lane 4, PS396 (1:200), Ser-396; lane 5, PS404 (1:200), Ser-404; and lane 6, PS413 (1:30), Ser-413. Use of PS396 (lane 7) that had been preabsorbed with its corresponding antigen peptide eliminated all reactivity.