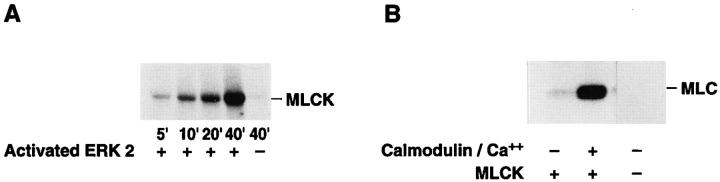
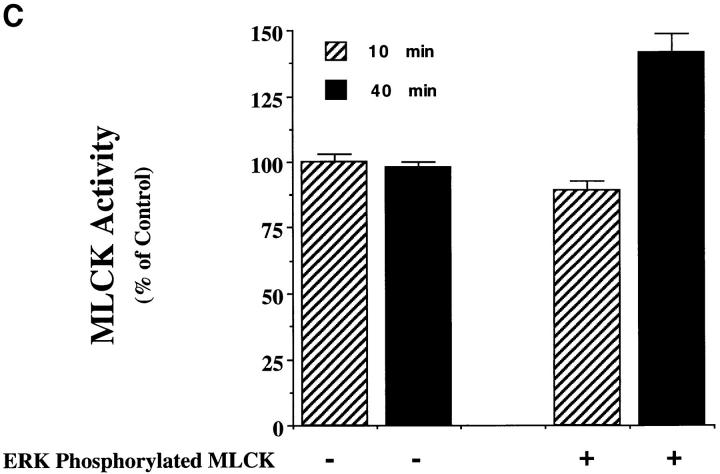
Figure 5.
MAP kinase directly phosphorylates MLCK, which leads to increased MLC phosphorylation in vitro. (A) Purified MLCK was monitored for phosphorylation in the presence or absence of purified activated MAP kinase for various times in an in vitro kinase assay as described in Materials and Methods. Note that there is no detectable phosphorylation of MLCK in the absence of MAP kinase. The result shown is a representative experiment from at least three independent experiments. (B) Purified MLCK phosphorylated by MAP kinase for 40 min was tested for its ability to phosphorylate MLC in an in vitro kinase assay in the presence or absence of Ca/calmodulin. Note that MLC was not phosphorylated by purified activated MAP kinase in these experiments. The result shown is a representative experiment from at least three independent experiments. (C) MAP kinase was allowed to phosphorylate MLCK as described in vitro for 10 or 40 min as described in Materials and Methods. The MLCK treated in the presence or absence of ERK was then allowed to phosphorylate MLC for 4 min in the presence of calcium and calmodulin as described in Materials and Methods. Data are expressed as percent of control MLCK activity (i.e., MLCK incubated for 10 min in the absence of ERK). Each point represents the mean ± SE of at least three experiments. The results are significant P < 0.005.