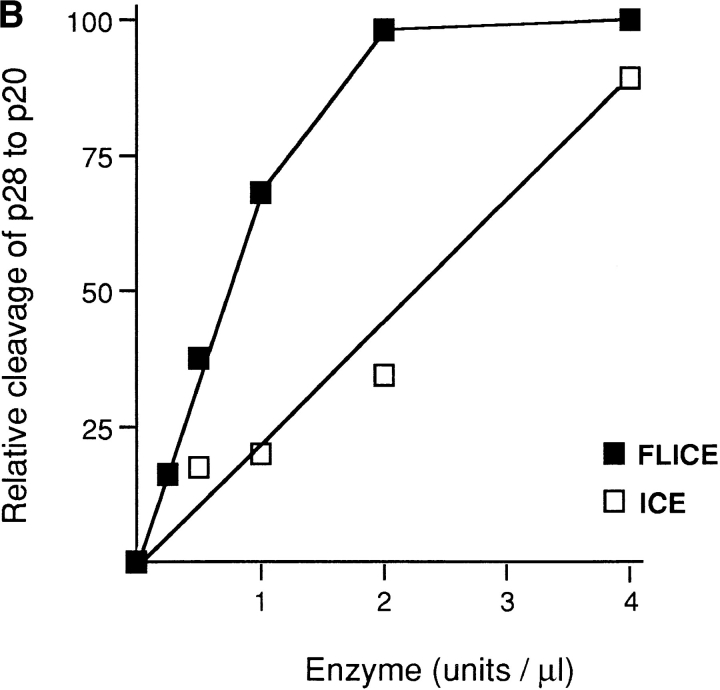
Figure 5.
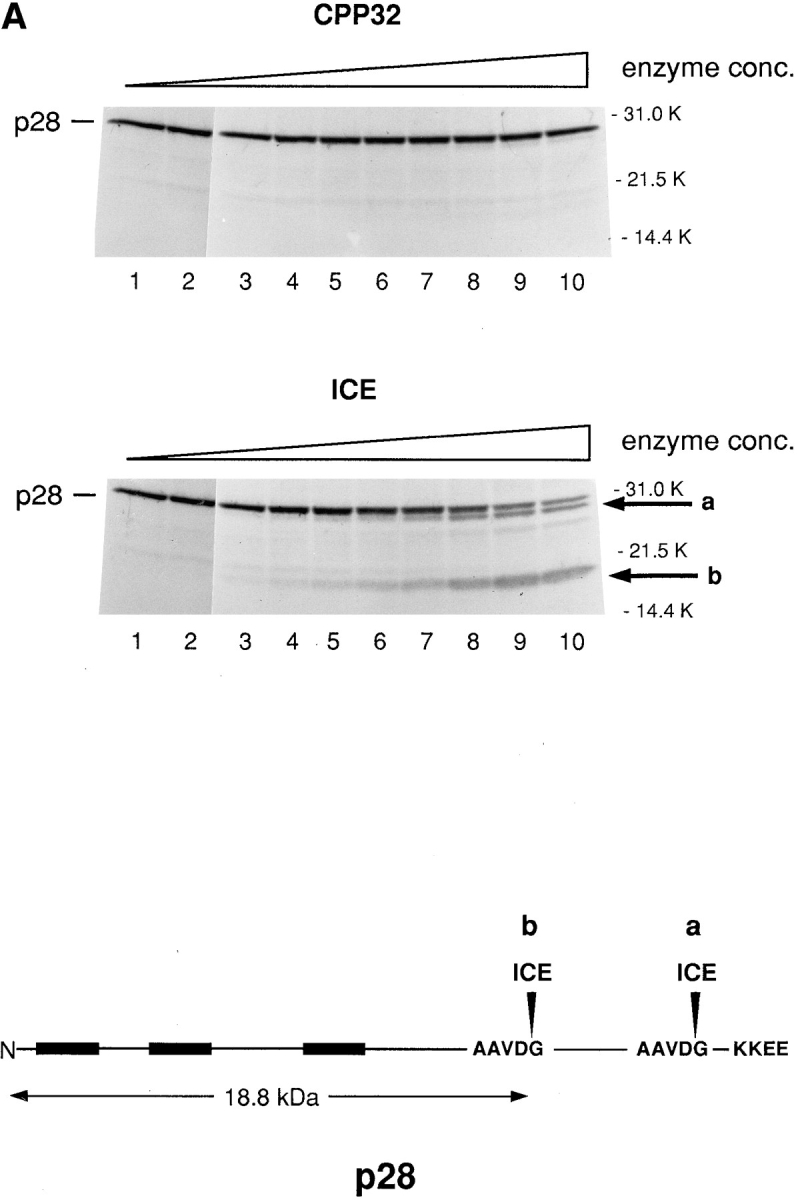
In vitro cleavage of p28 by caspase-1 (ICE) and caspase-8 (FLICE) but not by caspase-3 (CPP23). (A) 35S-labeled transcription–translation product of p28 cDNA was incubated with increasing concentrations of CPP32 or ICE, and the products were resolved by SDS-PAGE (Nicholson et al., 1995). Units of enzyme added per 25 μl reaction mixture were: none (lane 1), 0.0056 (lane 2), 0.98 (lane 3), 1.95 (lane 4), 3.9 (lane 5), 7.8 (lane 6), 15.6 (lane 7), 31.2 (lane 8), 62.5 (lane 9), and 125 (lane 10). The positions of polypeptide molecular mass markers are shown. The arrows designated a and b denote cleavage products whose sizes are consistent with cleavage of p28 at the sites indicated by a and b in the schematic at the bottom of the figure. (B) Same analysis as in A, except that p28 was incubated with purified ICE or FLICE, and the resulting p20 cleavage product was quantified using a Phosphorimager. 1 U of caspase enzyme activity is equivalent to 1 pmol aminomethylcoumarin liberated from fluorogenic tetrapeptide-AMC per min at 25°C at saturating substrate concentrations (Nicholson et al., 1995).