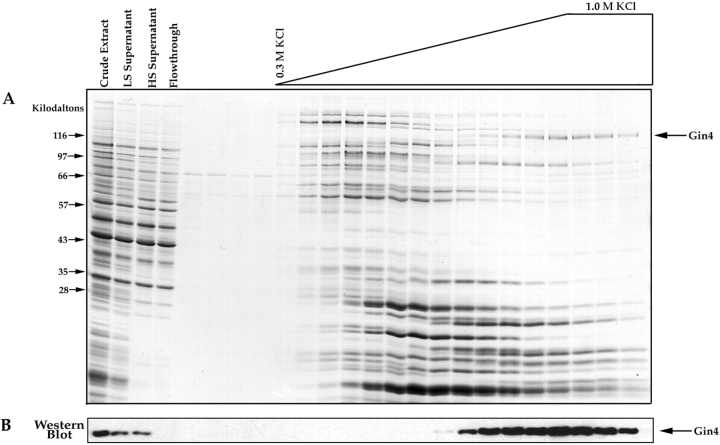
Figure 5.
Affinity purification of Nap1-binding proteins. (A) Crude extracts from log phase yeast cells were loaded onto a Nap1 affinity column. After washing with buffer, the column was eluted with a gradient of KCl, and samples from each fraction were precipitated with TCA and loaded onto a 12.5% SDS-polyacrylamide gel. The first few fractions before the start of the salt gradient show the final fractions of the wash. The gel is stained with Coomassie blue. (B) The same fractions shown in A were loaded onto a 10% SDS-polyacrylamide gel and transferred to nitrocellulose, which was then probed with an anti-Gin4 antibody. For the affinity column elution fractions we loaded 1/10 the amount of protein that was loaded onto the gel shown in A.