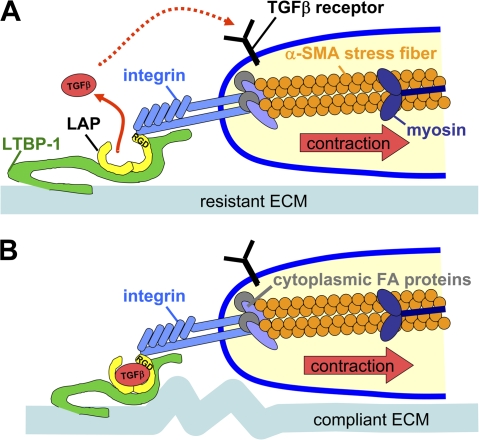
Figure 8.
Model of Mf contraction-mediated TGF-β1 activation. The high contractile activity generated by α-SMA in stress fibers is transmitted at sites of integrins binding to RGD sites in the LAP protein as part of the LLC, which also includes TGF-β1 and LTBP-1. (A) When the LLC is anchored in a comparably stiff ECM, cell-mediated stress can induce allosteric changes in LTBP-1 and/or LAP conformation, leading to liberation of TGF-β1; such activated TGF-β1 possibly feeds back by binding to its receptor, which is located close by in the activating cell. (B) In the context of compliant ECM, the LLC is dragged toward the pulling cell but because of the lack of mechanical resistance, no conformation change occurs and TGF-β1 remains latent. Likewise, inhibition of high cell contraction and interaction of integrins with the LLC block mechanical activation of latent TGF-β1.