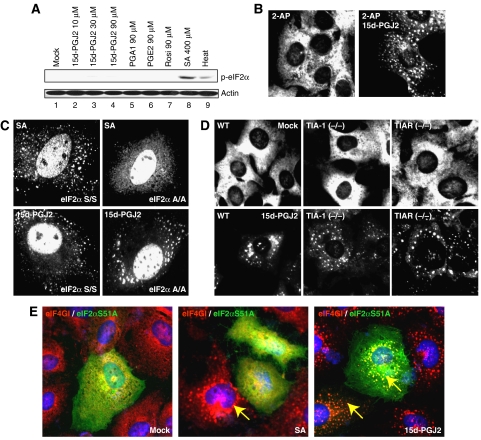
Figure 2.
SG formation by 15d-PGJ2 is independent of eIF2α phosphorylation and TIA-1 aggregation. (A) Phosphorylated eIF2α levels were monitored by western blot analyses using HeLa cell extracts (40 μg) treated with 15d-PGJ2 (lanes 2–4), PGA1 (lane 5), PGE2 (lane 6), Rosi (lane 7), or SA (lane 8) at the indicated concentrations for 30 min or with heat at 44°C for 30 min. (B) HeLa cells grown on cover slips were pretreated with 1 mM of 2-AP or with vehicle for 6 h, and then treated with 50 μM of 15d-PGJ2 for 30 min. Fixed cells were analyzed by immunocytochemistry with an eIF3b antibody. (C) The wild-type and eIF2α A/A mutant MEF cells were treated with 400 μM of SA for 30 min or 50 μM of 15d-PGJ2 for 1 h. Immunocytochemical assays were performed with a TIA-1 antibody. (D) The wild-type, TIA-1 KO, and TIAR KO MEF cells were mock-treated (upper panel) or treated with 15d-PGJ2 (lower panel). Immunocytochemical analyses were performed with an eIF3b antibody. (E) A plasmid encoding FLAG tagged-eIF2α S51A was transfected into HeLa cells. After 48 h of incubation, cells were mock-treated (left), treated with 400 μM of SA (middle) or with 50 μM of 15d-PGJ2 (right) for 30 min. The loci of eIF4GI and eIF2α S51A were visualized by an immunocytochemical method using eIF4GI and FLAG antibodies, respectively.