Figure 4.
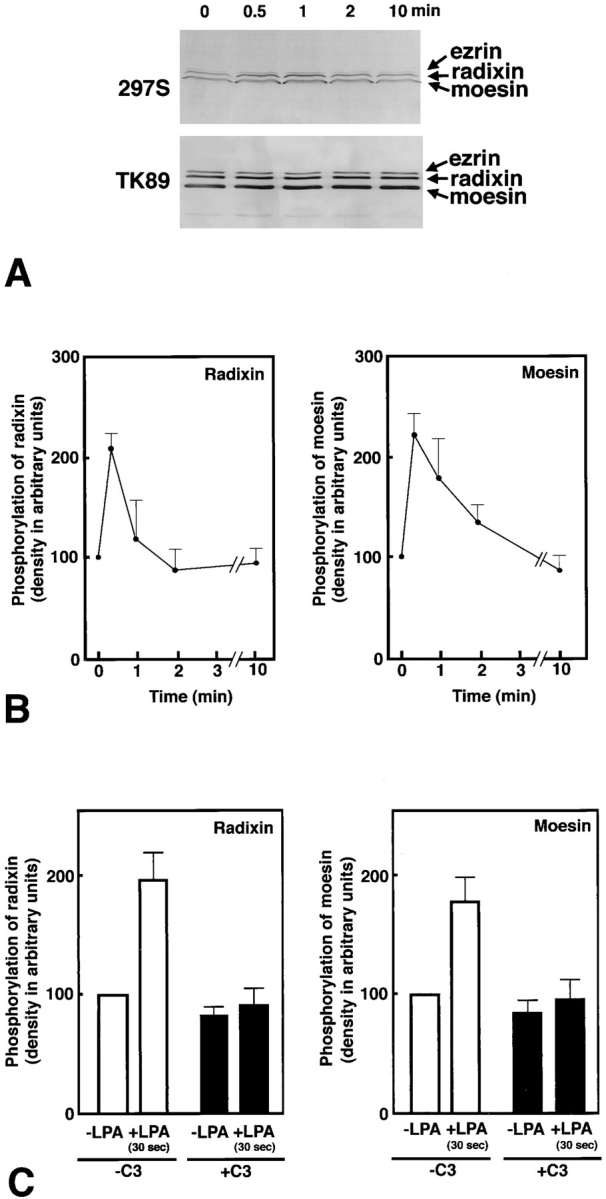
LPA-induced threonine phosphorylation of ERM proteins in vivo. (A) Serum-starved Swiss 3T3 cells were stimulated with LPA, and at 0, 0.5, 1, 2, or 10 min of incubation, whole-cell lysate was resolved by SDS-PAGE followed by immunoblotting with mAb 297S (297S) or with pAb TK89 (TK89). 297S were specific for T567-, T564-, and T558-phosphorylated ezrin, radixin, and moesin, respectively, whereas TK89 recognized ERM proteins irrespective of their phosphorylation state. (B) Quantitative analyses of changes in the phosphorylation levels of T564 in radixin and T558 in moesin. By immunoblotting with TK89 in combination with scanning densitometry, the precisely equal amount of ERM proteins were reelectrophoresed per each lane for the following quantification. The amount of T564-phosphorylated radixin and T558-phosphorylated moesin was quantitatively determined by scanning densitometry of 297S-immunoblots (see A) using purified phosphorylated C-rad to generate a standard curve. The curve was linear over the concentration range used here. The mean densities of 297S-immunoblot bands of radixin and moesin at 0 min were set at 100 arbitrary units. The phosphorylation levels of T564 in radixin and T558 in moesin rapidly increased then decreased within 2 min after LPA stimulation. Although the change in the phosphorylation level of T567 in ezrin was difficult to be quantitatively followed due to its low expression level in Swiss 3T3 cells, its phosphorylation level also appeared to rapidly increase then decrease within 2 min after LPA stimulation (see A). The data represent the means ± SEM of four determinations. The phosphorylation levels of radixin and moesin at 30 s were significantly different from those at 0 s (P < 0.005) and at 2 min (P < 0.05) as determined by the paired t test. (C) Suppression of LPA-induced T564 phosphorylation of radixin and T558 phosphorylation of moesin by C3 exoenzyme. Non- and C3 exoenzyme–pretreated serum-starved cells were stimulated with 1 μg/ml LPA for 30 s, and the phosphorylation levels of T564 of radixin and T558-moesin in these cells were quantitatively compared to those in LPA-nontreated serum-starved cells by immunoblotting with mAb 297S. The data represent the means ± SEM of four determinations. In the absence of C3 exoenzyme (-C3), the phosphorylation levels of radixin and moesin in 30 s LPA-treated cells were significantly different from those at 0 s (P < 0.05) as determined by the paired t test, while in the presence of C3 exoenzyme (+C3) this LPA-induced increase of the phosphorylation levels of radixin or moesin was not detected. In this experiment, probably due to lipofectamine, the Rho-dependent increase of ERM phosphorylation was varied and suppressed to some extent as compared to B.
