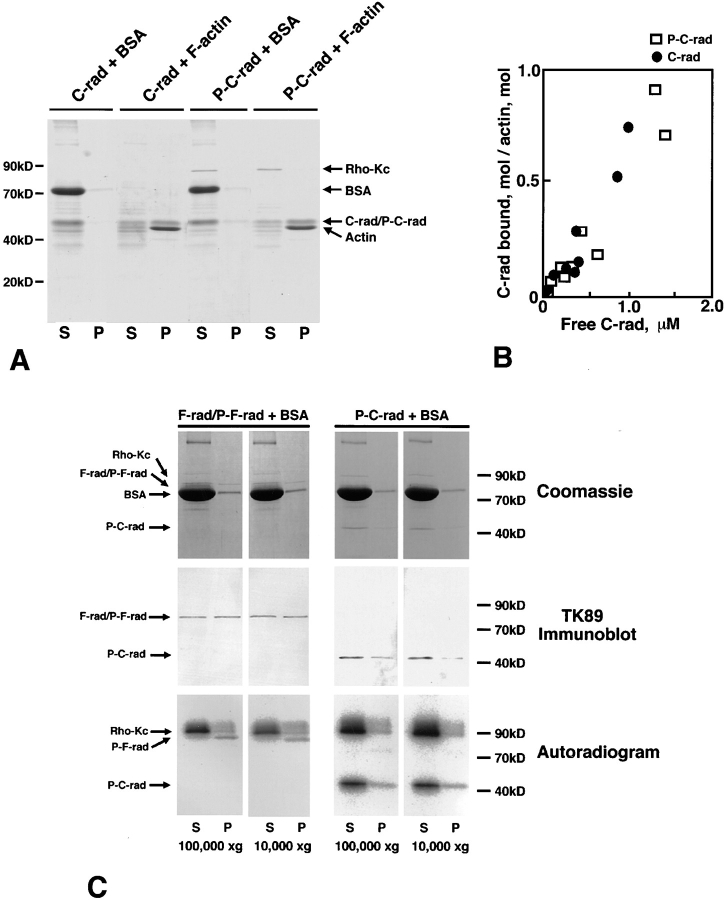
Figure 5.
Effects of T564 phosphorylation in radixin on the actin filament binding ability of C-rad. (A) Cosedimentation of non- or T564-phosphorylated C-rad with actin filaments. Non- (C-rad) or T564-phosphorylated C-rad (P-C-rad) was incubated with BSA (BSA) or with actin filaments (F-actin), then centrifuged at 100,000 g. Supernatant (S) and pellet (P) were resolved by SDS-PAGE followed by Coomassie brilliant blue staining. Both non- and T564-phosphorylated C-rad (C-rad/ P-C-rad) were cosedimented with actin filaments (Actin) to the same extent, but not with BSA (BSA). (B) Quantitative analysis. 2 μM F-actin was incubated with various amounts of non- or T564-phosphorylated C-rad and centrifuged, then the amounts of cosedimented non- (C-rad; filled circles) and T564-phosphorylated C-rad (P-C-rad; open squares) were quantified by densitometric scanning of Coomassie brilliant blue–stained gels. (C) Sedimentation of F-rad. Partially [32P]-phosphorylated F-rad (P-F-rad) and fully [32P]- phosphorylated C-rad (P-C-rad) were centrifuged in the presence of BSA at 100,000 or 10,000 g for 30 min. Supernatant (S) and pellet (P) were resolved by SDS-PAGE followed by Coomassie brilliant blue staining (Coomassie), immunoblot with TK89 (TK89 Immunoblot), or autoradiography (Autoradiogram). In the absence of actin filaments, F-rad, especially P-F-rad, were mostly recovered in pellet even at 10,000 g, whereas P-C-rad was mainly recovered in supernatant at 100,000 g as well as 10,000 g.