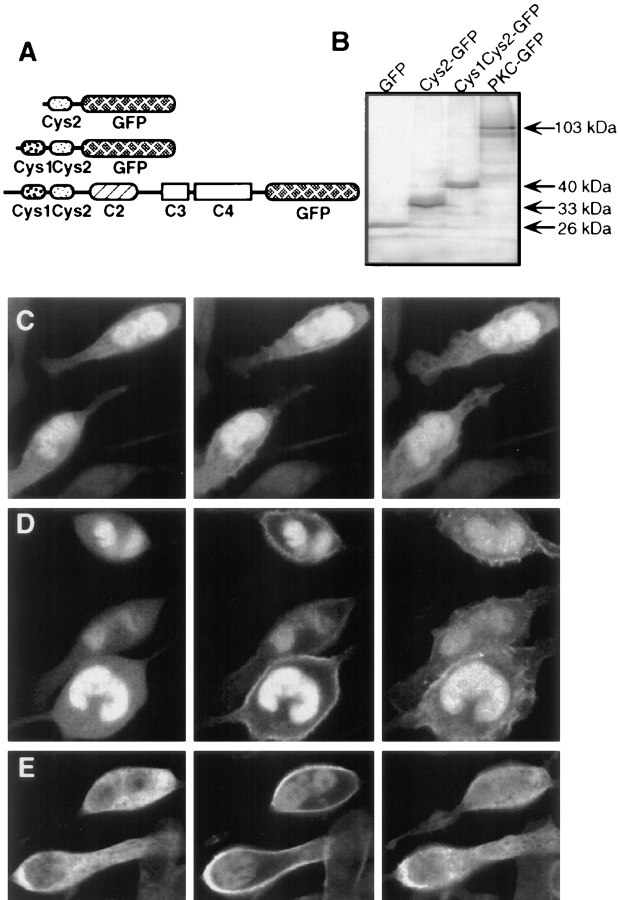
Figure 7.
Translocation of Cys2–GFP, Cys1Cys2–GFP, and full-length PKC-γ–GFP to the plasma membrane in response to receptor activation. (A) Schematic representation of the GFP-tagged constructs used in these experiments: Cys2–GFP, Cys1Cys2– GFP, and full-length PKC-γ–GFP. The proteins were expressed in RBL cells by microporation of in vitro transcribed RNA. (B) SDS-PAGE of [35S]Met-labeled proteins of in vitro transcribed GFP, Cys2–GFP, Cys1Cys2–GFP and PKC-γ–GFP. C–E represent series of three images of cells expressing the Cys2–GFP, Cys1Cys2–GFP and PKC-γ–GFP fusion proteins, respectively. The images were taken immediately before (left), 90 s after (middle), and 5 min after (right) stimulation with 100 nM PAF. Images were recorded at low laser intensity and were not corrected for photobleaching. A different plasma membrane translocation characteristic was observed for the three fusion proteins. Only a small fraction of the Cys2–GFP (D) translocated to the plasma membrane in response to receptor activation, while cytosolic Cys1Cys2–GFP (E) translocated more readily to the plasma membrane. Both, Cys2–GFP and Cys1Cys2–GFP, had also a typically higher concentration of the protein localized to the nucleus that was not significantly affected by receptor activation. (F) Full-length PKC-γ–GFP (E) showed significant nuclear exclusion in resting cells and a maximal transient localization to the plasma membrane in response to PAF receptor activation.