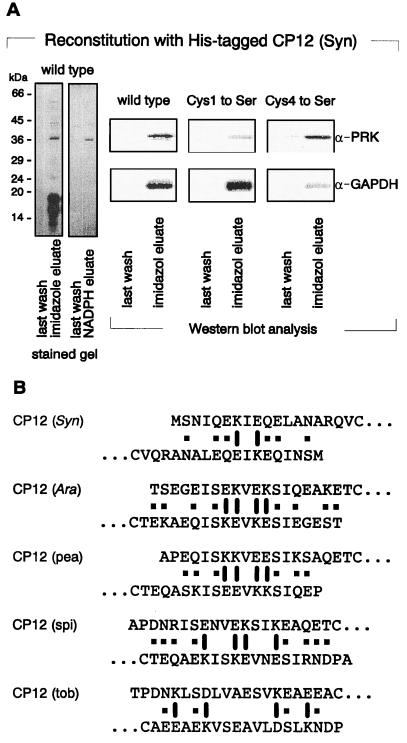
Figure 3.
Subunit composition and topology of the PRK/CP12/GAPDH complex of Synechocystis. (A) PRK binds to the N-terminal, GAPDH to the C-terminal peptide loop of CP12. Equal amounts of His-tagged, overexpressed and metal ion affinity purified CP12 of Synechocystis, as well as of the indicated cysteine for serine mutagenized and in E. coli overexpressed proteins, were added to aliquots of a DTT-reduced, soluble supernatant of lysed Synechocystis cells for in vitro complex reconstitution. After incubation, assay mixtures were chromatographed on metal ion columns. Bound proteins were eluted by either 100 mM imidazole or 2.5 mM NADPH in binding buffer, respectively, and subsequently characterized after SDS/PAGE by protein silver staining or Western blot analysis with monospecific antisera against PRK and GAPDH, as indicated. (B) CP12 might dimerize via charged amino acid residues located in its N terminus. N-terminal amino acid sequences up to the first cysteine of the so far known CP12 peptides of the indicated species are positioned in an antiparallel order. The resulting palindromic distribution of charged amino acid residues, thought to form salt bridges, is indicated by barrels. Amino acid residue pairs with the potential to form additional hydrogen bonds are marked by ■.