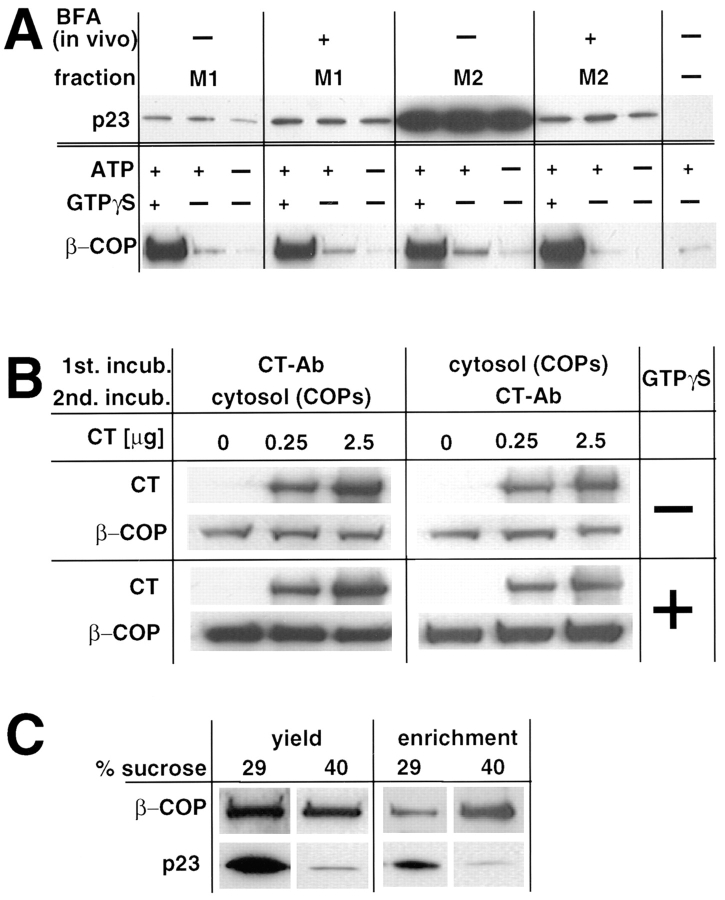
Figure 10.
In vitro recruitment of COP I. (A) Membranes with low (M1) and high (M2) p23 content were prepared by subcellular fractionation. When indicated (+BFA), cells were pretreated with BFA to shift p23 from M2 to M1 membranes upon fractionation. To recruit COP I onto membranes, fractions (25 μg) were incubated with cytosol (900 μg) in the presence of the indicated nucleotides. Membranes were sedimented, washed, and analyzed by Western blot with antibodies against the indicated proteins. (B) Membranes (25 μg protein) with high p23 content (M2) were sequentially incubated; first with increasing amounts of CT, and then with cytosolic COP I or vice versa. Membranes were sedimented, washed, and analyzed as in A, using antibodies against rabbit IgG (to reveal bound CT) or against β-COP. To quantitate the binding of whole anti-p23 IgG molecules (CT), samples were not boiled in SDS. Lower exposures of this Western blot did not reveal any differences between untreated and CT antibody– treated membranes (not shown). (C) COP-coated vesicles were generated in vitro from donor M2 membranes in the presence of cytosol, ATP and GTPγS. The mixture was then centrifuged to equilibrium on a sucrose gradient as in Sönnichsen et al. (1996), and fractions were collected. Membranes of the donor fraction (29% sucrose) and of the COP vesicles fraction (40%) were sedimented and analyzed with antibodies against p23 and β-COP. Analysis of equal volumes of each fraction (yield) reveals that the bulk of p23 does not partition into COP I–coated vesicles. Analysis of equal protein amounts (8 μg; enrichment) shows that β-COP is enriched, and p23 is de-enriched, in COP–coated vesicles.