Figure 3.
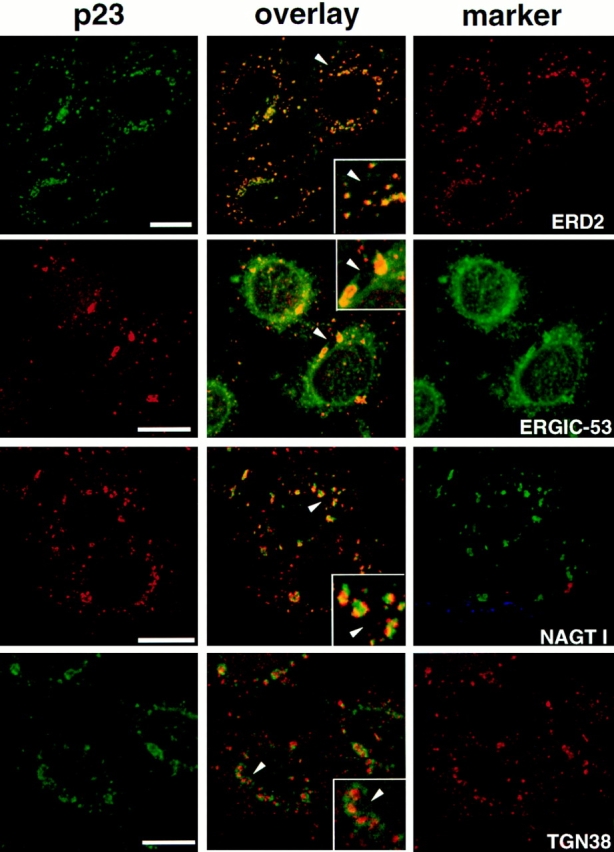
Immunolocalization of p23 after microtubule disruption with nocodazole. HeLa cells were treated with 10 μM nocodazole for 2 h to induce the formation of dispersed Golgi stacks, were fixed, and then processed for double immunofluorescence with antibodies against p23 and ERD2, ERGIC-53, myc-NAGT I, or TGN38. For double immunofluorescence of p23 with ERD2 or TGN38, biotinylated CT antibody was revealed with streptavidin-FITC. Fluorescein and rhodamine channels were merged (overlay) after adjustment of both fluorescence signals to similar levels. The inset shows a higher magnification of the area indicated by an arrowhead. The p23 signal overlapped completely with that of the KDEL receptor, ERD2, and extensively with that of ERGIC-53. Numerous ERGIC-53–positive membranes, corresponding to the ER, did not contain p23. Segregation of NAGT I and p23 occurred in perinuclear Golgi fragments that labeled for both markers, and in smaller peripheral structures that only labeled for p23. The absence of signal overlap between p23 and TGN38 was obvious. The colocalization of p23 with proteins of the cis-Golgi (ERD2), and the segregation of p23 and proteins of the medial (NAGT I) and trans-Golgi (TGN38) indicates that p23 localizes to the cis side of the Golgi. Bar, 5 μm.
