Fig 1.
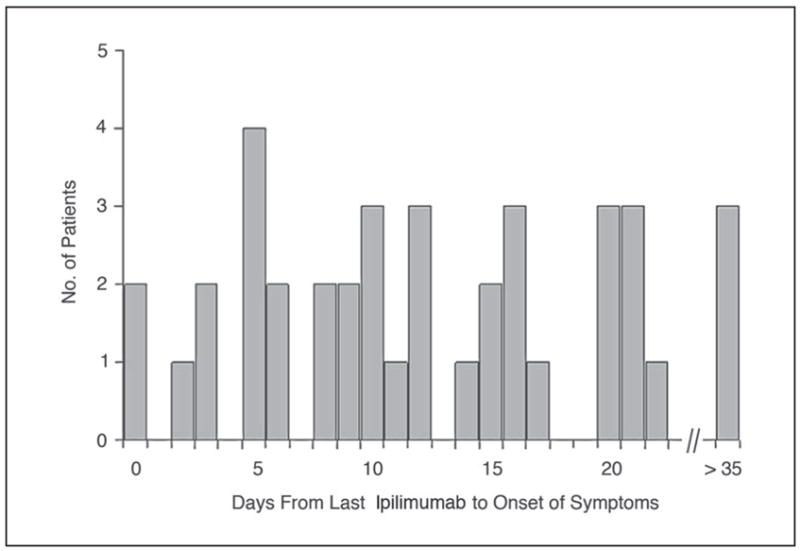
The interval from last dose of ipilimumab (MDX-010) to the onset of symptoms of enterocolitis. An interval of 0 days indicates a patient who received ipilimumab while having some symptoms, and those with intervals of more than 21 days had stopped ipilimumab dosing but went on to develop enterocolitis later.
