Fig.4. A protein of ∼40 kDa in size specifically interacts with the AG-rich region of tau exon 10.
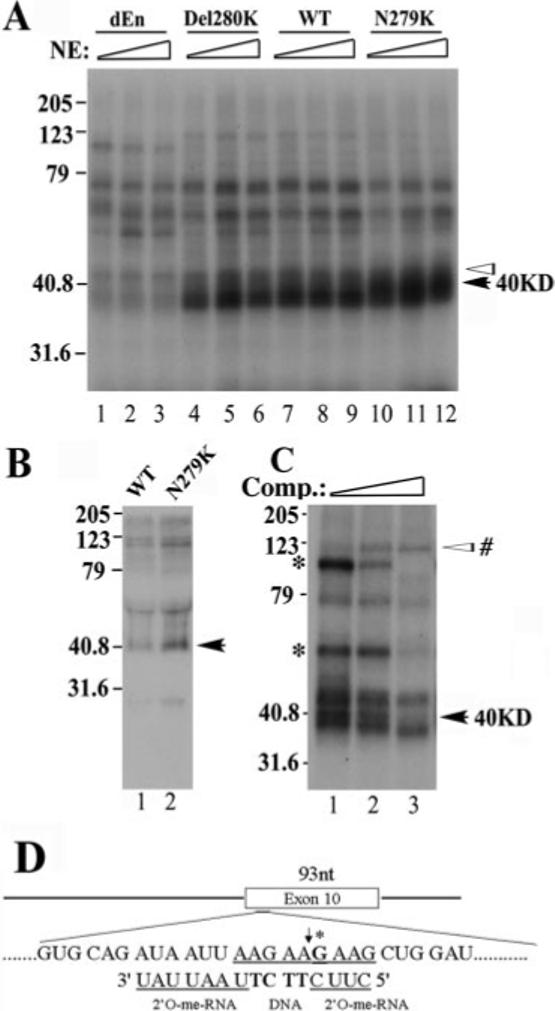
A, cross-linking of protein factors in the HeLa nuclear extract to Tau exon 10 RNA. Splicing reactions were performed as described with 32P-labeled RNA corresponding to exon 10 from dEn, Del280K, WT, and N279K constructs. Various amounts of HeLa nuclear extracts (10, 20, or 30 μg) were incubated with the RNAs under splicing conditions for 15 min. Aliquots of the reactions were UV-irradiated and then treated with RNase A. The cross-linking products were resolved on 12.5% SDS-PAGE and detected by autoradiography. The intensity of a 40-kDa protein (marked with a black arrow) correlates with the copy number of AAG repeats present in the AG-rich region of exon 10, whereas that of several other proteins (as indicated by white arrowheads) does not show such correlation. B, UV cross-linking/immunoprecipitation experiment. Tau exon 10 RNA corresponding to WT (lane 1) and N279K (lane 2) was used in a similar cross-linking experiment except that 1H4 anti-SR monoclonal antibody was added following the RNase A treatment to precipitate SR domain-containing proteins. Immunoprecipitated proteins were resolved on 12.5% SDS-PAGE and detected by autoradiography. C, the UV cross-linking experiments were carried out using N279K mutant RNA as shown in A except that the N279K RNA transcript used contained a site-specific 32P label in the AAG motif. The site-specific labeled G is marked with an asterisk in D. The chimeric oligonucleotide used for RNase H cleavage to generate the half-RNAs is also indicated in D. The cross-linking experiment was done in the presence of increasing amounts of competing oligonucleotide corresponding to the AAG-rich enhancer region. Molecular weights are shown on the left sides of gels (in kDa). The black arrows on the right mark the position of the 40-kDa protein. In C, an asterisk marks the cross-linking species that remain to be investigated.
