Fig.5. Tra2β protein interacts with the Tau exon 10 AG-rich region.
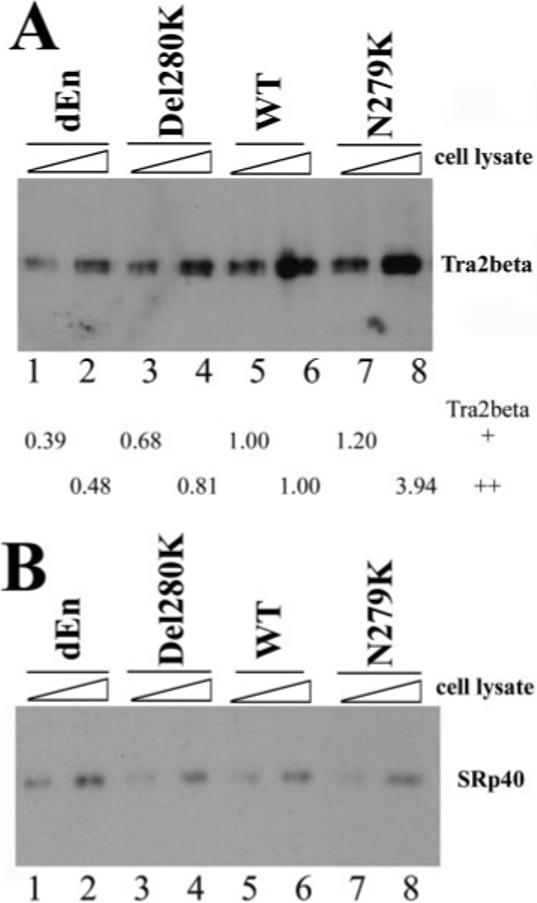
40 fmol of biotinylated TauEx10−11 RNAs corresponding to dEn, Del280K, WT, and N279K (with zero, one, two, and three copies of AAG repeats, respectively) were incubated on ice for 30 min in 25-μl reactions under splicing conditions with increasing amounts of cellular extract (0.5 or 1 μl) containing transiently transfected Myc-tagged Tra2β (A) or HA-tagged SRp40 (B). After affinity selection with streptavidin-agarose beads, the bound proteins were detected by Western blotting using anti-Myc antibody (for Tra2β-Myc) or anti-HA antibody (for SRp40). The intensity of Tra2β protein pulled down by dEn, Del280K, or N279K RNA in A was quantified, respectively, and compared with that pulled down by WT RNA. The numbers shown below the gel in A represent the relative quantification using the band intensity in the wild type (lane 5 or lane 6, respectively) as 1.00 in the presence of either 0.5 μl (lanes 1, 3, 5, and 7) or 1 μl (lanes 2, 4, 6, and 8) of Tra2β-expressing cell lysates.
