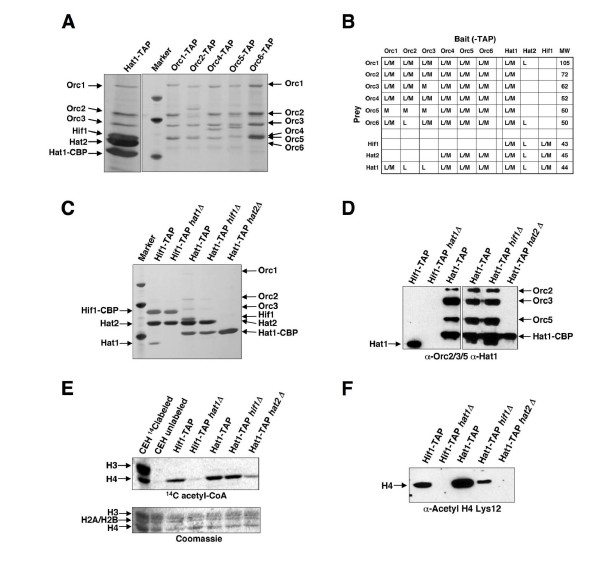
Figure 1.
Physical interaction of ORC with the Hat1p/Hat2p complex. (A) The B-type HAT complex was purified from a strain containing a TAP-tagged Hat1p subunit, and ORC was purified from strains carrying either a TAP-tagged version of Orc1p, Orc2p, Orc4p, Orc5p, or Orc6p. For comparison, lane 2 shows marker proteins (MWs are 45.0, 66.2, and 97.4 kDa). The positions of the respective subunits of the ORC and Hat1p complex are indicated. Hat1-CBP: Hat1p with calmodulin binding protein (CBP) after TEV digestion of the TAP-tag. (B) Summary of proteins identified in purifications of TAP-tagged baits. Components of ORC and Hat1p complexes that were detected at least once with either MALDI-TOF or liquid chromatography-mass spectrometry (LC-MS) with high confidence (>90%) are indicated (M/L). (C) Architecture of the Hat1p complexes by differential tagging and subunit deletions. Strains were HIF1-TAP (BSY675), HIF1-TAP hat1Δ (BSY681), HAT1-TAP (BSY679), HAT1-TAP hif1Δ (BSY720), HAT1-TAP hat2Δ(BSY682). Lane 1 shows marker proteins (45.0, 66.2, and 97.4 kDa). (D) Western blot from two series of TAP purifications (Figure 1C for left panel, Additional file 1 for right panel), probed with a-Hat1p, a-Orc2p, a-Orc3p, and a-Orc5p antibodies. (E) In vitro histone acetyltransferase activities of Hat1p complexes. Concentrated eluate (10-fold) from indicated TAP-tag purifications from Figure 1C was used for HAT-assays with 14C acetyl-CoA and chicken erythrocyte histones. The upper panel shows 14C incorporation into histones shown in the lower panel by Coomassie strain. (F) In vivo association of acetylated histone H4 with Hat1p sub-complexes from Figure 1C). Eluates from TAP-tag purifications were analyzed for Lys12 acetylated histone H4 by Western blot (a-Acetyl H4 Lys12).