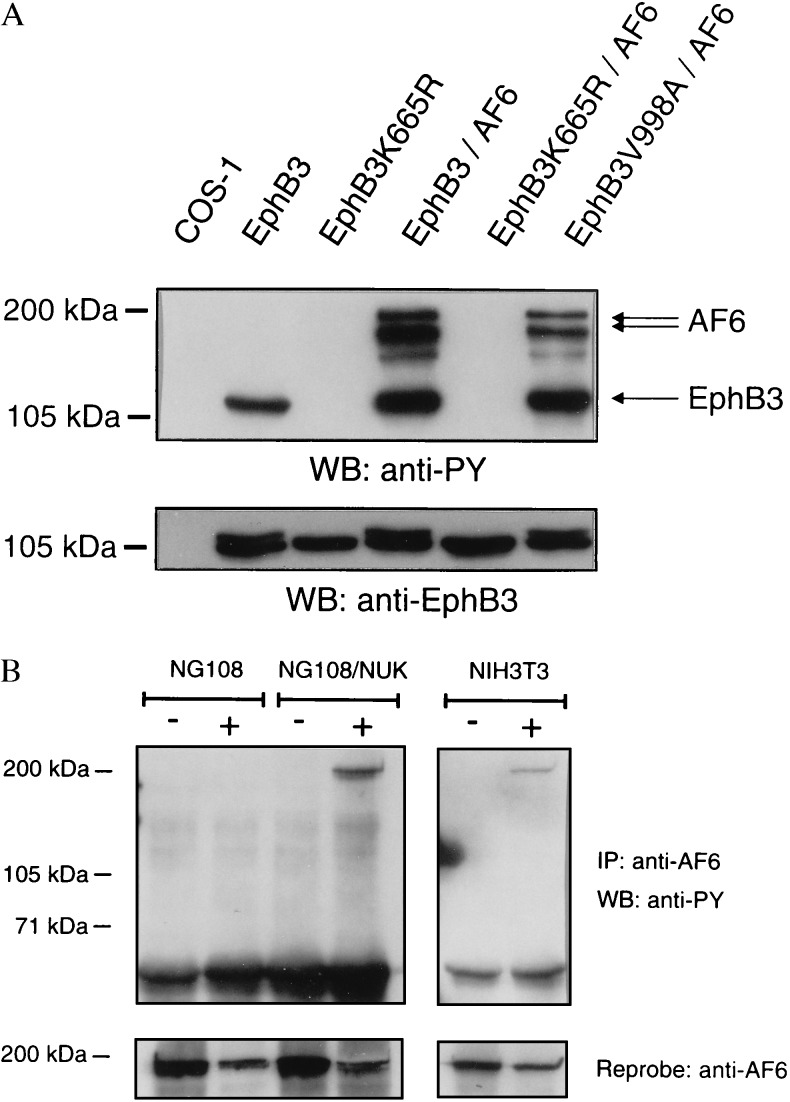
Figure 3.
Phosphorylation of full-length AF6 depends on the catalytic activity of the receptor. (A) COS-1 cells were cotransfected with various mutants of EphB3 and HA-AF6. Cell lysates were probed on a Western blot with phosphotyrosine antibodies. AF6 is recognized as multiple bands presumably due to degradation of the overexpressed protein. The EphB3 and HA-AF6 content of the lysates was compared on separate blots using HA (not shown) and EphB3 antisera (Lower). (B) NIH 3T3, NG108, or NG108 cells stably expressing EphB2 (NG108/NUK) were challenged with 2 μg/ml clustered ephrine-B1 Fc (+) or human Fc antibodies (Sigma) for 30 min (−). Subsequently, cells were scraped off, washed twice in PBS, and lysed in RIPA buffer. Cell lysates were immunoprecipitated (IP) with a polyclonal AF6 antiserum and probed on a Western blot (WB) using monoclonal phosphotyrosine antibodies (PY99, Santa Cruz Biotechnology), stripped, and reprobed with AF6 antibodies.