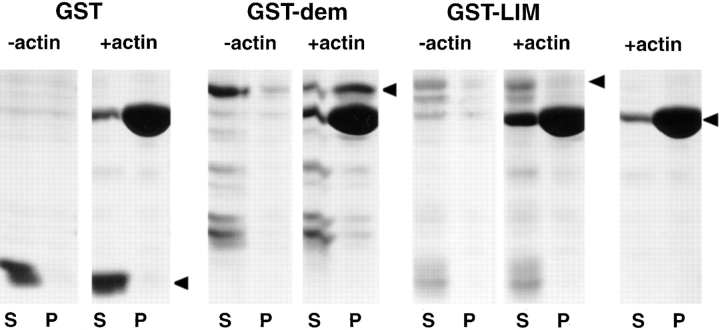
Figure 6.
Actin binding to GST–abLIM fusion proteins. Three different polypeptides expressed in E. coli and purified by affinity chromatography were tested for the ability to associate with F-actin: GST only (GST), GST fused to part of the dematin region of abLIM (GST–dem), and GST fused to part of the LIM domain of abLIM (GST–LIM). Each polypeptide was incubated with (+actin) or without (−actin) actin under conditions that favor actin polymerization. After centrifugation to sediment F-actin, each sample was separated into a supernatant (S) and pellet (P). Samples are indicated at the top of the SDS gel. Control incubations with actin but without added fusion proteins were run in parallel (far right panel). Mobilities of each fusion protein with the appropriate mass are indicated by filled arrowheads (right). Note the presence of each fusion protein in the supernatant fraction in −actin samples (left). Only GST–dem is enriched in the +actin pellet fraction, indicating cosedimentation of GST–dem, but not GST or GST–LIM, with F-actin.