Abstract
Background
The incidence of salivary gland tumors is claimed to be influenced by geographical and racial factors. The pathological classification and nomenclature of salivary gland tumors as defined by WHO classification (1991), is accepted world-wide but little is available in the literature regarding the spectrum of salivary gland tumors in Africa in the basis of this classification. Such efforts would allow comparison and justify any differences between the black African population and the rest of the world.
Objective
To outline the clinicopathological features of salivary gland tumors in Uganda.
Setting
Makerere University, Faculty of Medicine, Department of Pathology.
Methods
All epithelial tumors from major and minor salivary glands accessioned from 1979 to 1988 were analyzed in respect to sex and age of patients, anatomical location of the tumor and histological type. The histological diagnosis of each individual tumor was based on the 1991 WHO classification of salivary gland tumors.
Results
During the span of 10 years, 268 cases of salivary gland tumors were diagnosed. Of these, 113 (42.2%) were males, 148 (55.2%) females and in the remaining seven (2.6%) cases, the sex was not specified. The age range of the 247 patients with recorded ages was from 0.5 to 80 years. The mean age at diagnosis was 38.1 (SD =17.03) with the median of 38.0 years. Thirty four percent of tumors originated from the parotid, 33.2% from the submandibular and 32.8% from minor salivary glands. No tumor was implicated from the sublingual gland. There were a total of 125 (46.6%) malignant tumors and 143 (53.4%) benign tumors. The mean age of patients with malignant lesions (43.1 years; SD=16.75; median=44.00 years) was 9.6 years older than those with benign tumors (mean=33.5 years; SD=16.0; median=30.00 years). Pleomorphic adenoma was the most common benign tumor (74.8%), followed by myoepithelioma (9.8%). No Whartin's tumor was encountered. The malignant tumors were dominated by adenoid cystic carcinoma (28.8%) followed by mucoepidermoid carcinoma (21.6%).
Conclusion
The pattern of distribution of salivary gland tumors in black African population seems to differ from that of Western series in that; i) females are more affected than males, ii) there is a low proportion of tumors from the parotid gland and high proportion of tumors from the submandibular and minor salivary glands, iii) the parotid and minor salivary gland tumors have more probability of being malignant than those tumors from the submandibular gland iv) the newly categorized pathological entities are common and v) Whartin's tumor is extremely rare in black African population.
Introduction
Neoplasms of salivary glands though uncommon, are of particular interest to both histopathologists and surgeons because of their varied histological and biological characteristics and the difficulties involved in management. This diverse group of tumors with wide spectrum of biologic activity has long natural history with many associated factors that make them a therapeutic challenge. Malignant and benign salivary gland tumors may resemble each other grossly if seen early in their clinical course. Furthermore, the histological bland nature of most malignant salivary gland tumors such as basal cell adenocarcinoma (BCAC), low grade mucoepidermoid carcinoma, acinic cell carcinoma and others, and the aggressive biological nature of benign tumors as typified by high rate of recurrence and repeated surgical failures in pleomorphic adenoma and myoepithelioma, may easily lead to misdiagnosis and consequently contribute to complexity of their management. In view of the fact that these tumors are treated on the basis of their histological and local findings, correct histological diagnosis is obligatory. Accurate histological diagnosis is dependant on clearly defining the histological cell type and morphological patterns which is the basis of the 1991 WHO classification of salivary gland tumors.
Literature from various parts of the world1–4 including the one from Uganda by Davies et al. (1964)5, point out that, there are differences in the frequency of particular histological types and in the frequency with which major and minor salivary glands are involved. However, most of these reports were published prior to the present WHO classification6 which due to variations in terminology, hinder direct comparisons between one series and another. For example, the so-called ‘basal cell type of mixed tumor’ as illustrated by Schulenburg7 would according to present WHO classification be described as basal cell adenoma or basal cell adenocarcinoma, depending on its infiltrative nature. In Uganda, since Davies5 and his co-workers published an outstanding report on salivary gland tumors in 1964, to the knowledge of the author, no other report in the English literature is available that has appraised the findings recorded by then. With this in mind, this investigation was undertaken to analyze the clinico-pathological features of 268 salivary gland patients from the Department of Pathology at Makerere University, Uganda during the period between 1979 to 1988.
Methods
Study subjects
Hematoxylin and Eosin stained slides from the files of the Department of Pathology, Makerere University-Uganda, were retrieved and re-examined. These included 268 consecutive cases of primary salivary gland tumors from black African patients from year 1979 to 1988. Tumors from lacrimal glands, nose and nasal sinuses were excluded. Several anaplastic carcinomas from minor salivary glands lacking glandular differentiation were excluded because it could not be ascertained whether they were of salivary gland origin or from oral mucosal epithelium. Also excluded were all non-epithelial tumors from patients who were seen because of salivary gland swelling.
Clinicopathological variables
The clinical and pathological information retrieved from patients files included; sex, age, histological diagnosis and anatomical location of the tumor. All histological specimens of each tumor were microscopically examined and classified according to the 1991 WHO Classification of salivary gland tumors6.
Results
Age and sex
The age at presentation and sex distribution is shown in Table 1. The ages at initial histological diagnosis of 247 patients with recorded ages, ranged from 0.5 to 80 years (mean 38.1 years; SD=17.0; median 38.0 years). About ninety percent (90.8%) of patients were between 2nd and 6th decades. There were 113 (43.3%) males and 148 (56.7%) females with male to female ratio of 1:1.3 for all tumors. The sex was not recorded in seven cases. Overall, no difference in mean age was noted between female (mean=37.9 years; SD=15.0: median=38.0), and male (mean=38.4 years; SD=18.4; median= 39.0) patients, except for male patients with malignant tumors who were somewhat older (mean=44.5; SD=18.1; median=41.0 years) than their female counterparts (mean=41.6; SD=15.0; median=42.0 years). However, the mean age of patients with malignant tumors (mean=43.1 years; SD=16.8; median=44.0 years) was much higher than the mean age (mean 33.5 years; SD=15.1 years; median=30.0 years) of patients with benign tumors by 9.6 years.
Table 1.
Age in years and sex distribution of total cases of salivary gland tumors
| Age | Males | Females | Not stated | Total |
| 0–10 | 2 | 1 | - | 3 |
| 11–20 | 21 | 23 | - | 44 |
| 21–30 | 23 | 30 | - | 53 |
| 31–40 | 11 | 25 | - | 36 |
| 41–50 | 25 | 29 | - | 54 |
| 51–60 | 15 | 24 | - | 36 |
| 61–70 | 6 | 5 | - | 11 |
| 71–80 | 7 | 2 | - | 9 |
| Unknown | 3 | 9 | 7 | 19 |
| Total | 113 | 148 | 7 | 268 |
| Mean age | 38.6±18.4 | 37.9±16.0 | - | 38.1±17.0 |
| Median | 39.0 | 38.0 | - | 38.0 |
| Age range | 0.5–79 | 10–80 | - | 0.5–80 |
Table 2 shows the distribution of malignant and benign tumors by sex. There were 123 (46%) malignant and 145 (54%) benign tumors with malignant to benign ratio of 1:1.18. Excluding the seven cases with unrecorded sex, there was a female predominance in both malignant (64/122=52.4%) and benign (84/139=60.4%) tumors. However, proportional-wise, about half (58/113=51.3%) of all male patients were diagnosed with malignant neoplasm against the 43.2% (64/148) of all female patients (Table 2). In both sexes parotid and minor salivary glands were more likely to have malignant neoplasms. Of all males with parotid gland tumors, nearly 60% of them had malignant lesions, while this phenomenon was encountered in two thirds of tumors from minor salivary gland. About 50% of females with parotid and minor salivary gland tumors had malignant tumors.
Table 2.
Distribution of benign and malignant tumors in respect to site and sex
| Site | Males | Females | Not Stated | |||||
| Benign | Malignant | %* | Benign | Malignant | %* | Benign | Malignant | |
| Parotid | 15 | 22 | 59.4 | 27 | 27 | 50.0 | - | - |
| Submandibular | 27 | 10 | 27.0 | 35 | 7 | 32.7 | - | - |
| Sublingual | - | - | - | - | - | - | - | - |
| Minor glands | 13 | 26 | 66.7 | 22 | 20 | 47.6 | 6 | 1 |
| Total | 55 | 58 | 51.3 | 84 | 64 | 43.2 | 6 | 1 |
Site
The percentage frequency of total salivary gland tumors in various sites is illustrated in Table 3. Although there was a slightly preponderance of parotid tumors (34%), the distribution is similar to that of submandibular (33.2%) and minor salivary glands (32.8%). Of the latter site, about 50% (43 tumors) arose from the palate. Other locations of minor glands included, cheek, (17), lip (4), tongue (7), retromolar region (6) and maxillary sinus (4). In seven intraoral tumors the site was not exact. More than half of the tumors from the parotid (54%) and those from minor glands (53%) were diagnosed as malignant. Noticeably, no involvement of sublingual gland was recorded in this series.
Table 3.
Frequency of benign and malignant salivary gland tumors in respect to site
| Absolute No. | Benign | Malignant | ||||
| Site | No. | % | No | % | No. | % |
| Parotid | 91 | 34.0 | 42 | 46.2 | 49 | 53.8 |
| Submandibular | 89 | 33.2 | 62 | 69.7 | 27 | 30.3 |
| Sublingual | - | - | - | - | - | - |
| Minor glands | 88 | 32.8 | 41 | 46.9 | 47 | 53.4 |
| Total | 268 | 100.0 | 145 | 54.1 | 123 | 45.9 |
Histological types and their distribution
Benign tumors
The most frequent histological diagnosis was pleomorphic adenoma (73.8% of all benign tumors (Table 4) and 40% (107/268) of all tumors) in the series. The second most common benign tumor was myoepithelioma (11.2%), followed by basal cell adenoma (6.2%), both of which are newly defined entities. The rarely encountered tumors such as oncocytoma and cystadenoma were not infrequently identified in this series (Table 4). The most affected site for benign tumors was the submandibular gland (43%). The parotid (29%) and minor glands (28%) had equal distribution of benign neoplasm. No Whartin's tumor was found in this series.
Table 4.
Distribution of benign tumors by site
| Site | ||||||||
| Parotid | Submandibular | Minor | Total | |||||
| Histological types | No. | % | No. | % | No. | % | No. | % |
| Pleomorphic adenoma | 31 | 73.8 | 48 | 77.4 | 28 | 68.3 | 107 | 73.8 |
| Myoepithelioma | 3 | 7.1 | 5 | 8.1 | 8 | 19.5 | 16 | 11.0 |
| Basal cell adenoma | 4 | 9.5 | 4 | 6.5 | 1 | 2.4 | 9 | 6.2 |
| Oncocytoma | 4 | 9.5 | 2 | 3.2 | 1 | 2.4 | 7 | 4.8 |
| Csytadenoma | - | - | 3 | 4.8 | 3 | 7.4 | 6 | 4.1 |
| Total | 42 | 28.9 | 62 | 42.8 | 41 | 28.3 | 145 | 100.0 |
Malignant tumors
Adenoid cystic carcinoma was the most encountered malignant (29%) tumor and was second (13.4%) after pleomorphic adenoma in a whole series. Fifty percent of adenoid cystic carcinoma originated from minor glands of the oral cavity. It was followed by mucoepidermoid carcinoma (20.3%), and acinic cell carcinoma (13.1%), (table 5). The newly categorized entities such as polymorphous low grade adenorcacinoma (PLGA), epithelialmyoepithelial carcinoma (EMC), basal cell adenocarcinoma (BCAC) and malignant myoepithelioma, were also identified in this series (Table 6). Only 22% of all malignant tumors arose from the submandibular gland, whereas, 40% and 38% of malignant tumors were from the parotid and minor salivary glands, respectively.
Table 5.
Distribution of malignant tumors by site
| Site | ||||||||
| Histological diagnosis | Parotid | Submandibular | Minor glands | Total | ||||
| No. | % | No. | % | No. | % | No. | % | |
| Adenoid cystic carcinoma | 8 | 16.3 | 10 | 37.0 | 18 | 38.3 | 36 | 29.3 |
| Mucoepidermoid carcinoma | 11 | 22.5 | 5 | 18.5 | 9 | 19.2 | 25 | 20.3 |
| Acinic cell carcinoma | 8 | 16.3 | 3 | 11.1 | 5 | 10.6 | 16 | 13.0 |
| Epithelial-myoepithelial carcinoma | 1 | 2.1 | 3 | 11.1 | 2 | 4.3 | 6 | 4.9 |
| PLGA | 1 | 2.1 | 2 | 7.4 | 7 | 14.9 | 10 | 8.1 |
| Basal cell adenocarcinoma | 3 | 6.1 | - | - | - | - | 3 | 2.1 |
| Adenocarcinoma | 7 | 14.2 | - | - | 3 | 6.4 | 10 | 8.1 |
| Carcinoma in pleomorphic adenoma | 3 | 6.1 | 1 | 3.7 | 2 | 4.3 | 6 | 4.9 |
| Malignant myoepithelioma | 2 | 4.1 | 2 | 7.4 | 1 | 2.1 | 5 | 4.1 |
| Squamous cell carcinoma | 3 | 6.1 | 1 | 3.7 | - | - | 4 | 3.3 |
| Undifferentiated carcinoma | 2 | 4.1 | - | - | - | - | 2 | 1.6 |
| Total | 49 | 39.8 | 27 | 22.0 | 47 | 38.2 | 123 | 100.0 |
Table 6.
Percentage of Salivary gland tumors at different sites in series from several country
| Series | No of cases | Parotid | Subman dibular |
Sublingual | Minor glands |
Unknown |
| Present series | 268 | 33.9 | 33.2 | - | 32.8 | - |
| Davies et al. 1964, Uganda | 129 | 52.7 | 19.4 | 2.3 | 27.9 | 2.3 |
| Thomas et al. 1980, Malawi | 190 | 43.7 | 24.2 | 0.5 | 32.0 | 0.5 |
| Onyango et al. 1992, Kenya | 417 | 31.9 | 18.5 | 0.5 | 45.8 | 3.3 |
| Schulenburg 1954, South Africa | 105 | 46.0 | 19.0 | - | 31.0 | 4.0 |
| Ezeanolue 1999, Nigeria | 41 | 60.9 | 24.4 | - | 14.6 | - |
| Ouoba et al. 1998, Dakar | 48 | 66.7 | 20.8 | - | 12.5 | - |
| Arotiba 1996, Nigeria | 237 | 32.1 | 19.4 | - | 24.9 | - |
| El-Gazayerli et al.1964, Egypt | 78 | 47.4 | 15.4 | - | 37.2 | - |
| Nagler et al. 1997, Israel | 245 | 57.9 | 13.9 | 0.8 | 27.3 | - |
| Yu et al. China | 405 | 44.8 | 12.8 | 0.5 | 37.3 | - |
| Eveson 1985, UK | 2410 | 72.9 | 10.9 | 0.3 | 14.0 | - |
| Morgan et al. 1968, UK | 244 | 71.3 | 9.1 | - | 19.7 | - |
| Davies et al. 1964, UK | 80 | 75.0 | 13.7 | - | 11.3 | - |
| Sharkey 1977, USA | 366 | 86.1 | 6.3 | 0.3 | 7.1 | 0.5 |
Discussion
The categorization of salivary gland tumors as suggested by WHO pathological classification6, has proved to be most useful in daily practice and has been adopted worldwide1. The nomenclature assists in further understanding of the biological behavior of these neoplasms in relation to their histological patterns. This classification forms the basis of this study.
The findings from this study substantiate the earlier reports that there are a number of clinicopathological differences between the salivary gland tumors in black population and those from white populations of Europe and United States4,5,7–11.
Traditionally, the distribution of salivary neoplasms between sites has followed a rule of 100:10:10:1 ratio for parotid, submandibular, minor salivary glands and sublingual tumors, respectively. This general principle which is formulated as a result of accumulated data from non-African series1–3,12–14 appears to be inappropriate for African population. In this study there was nearly a 1:1:1 ratio of tumors from the parotid (34%), submandibular (33%), and minor salivary glands (32%), in that order. This is in agreement with recent work from Congo (Zaire)8 and Nigeria10 that has reported a parotid gland involvement of 36.6% and 32.1%, respectively. Similar experience has been documented in previous reports from Malawi4, Uganda5, South Africa7, Kenya9, and Egypt11.
This peculiar relatively low incidence of salivary gland tumors of the parotid gland and relatively high incidence of the submandibular and minor salivary gland in comparison to the western series is worthy of comment. There appears to be consistently different pattern of presentation between African population and those of Western world. Although the explanation for this is not clear, some workers have questioned whether endemic parotitis may not diminish the susceptibility of the parotid gland to neoplasm or increase the susceptibility of the submandibular to it11. However, other researchers have argued in respect to the nature of serous cells in the parotid gland. El-Gazayerli11 contents that in the studies of the changes in salivary glands and malnutrition, serous cells are more severely affected than the mucous cells. This selective partiality of the serous cells in malnutrition may explain the altered parotid to submandibular ratio by diminishing the susceptibility of the parotid gland to neoplasm. A similar low parotid involvement has been reported from non-Western non-African population such as, Israel12 and China13. Further work on the relationship between endemic parotitis that is usually associated with malnutrition should be done in regard to neoplastic changes occurring in salivary gland tissue.
No tumor from sublingual gland was recorded from this study. Sublingual gland is unusual site for tumors in both Western and African series. The reported incidence is less than 0.5% of all salivary gland tumors.
A significant proportion (46%) of malignant tumors was seen in this series. In Thomas et al.4 publication, a third of all tumors were malignant, and Morgan and co-authors14 reported a malignant frequency of 32.4%. Seifert et al1. with a sample size of 2579 cases reported a frequency of 25.7% while Ouoba et al15., with a small sample size of 48 cases, reported malignancy rate of 16.7%. Onyango and collegues9 with adequate study sample of 417, account malignancy frequency of 27.6%. The discrepancy may be displaying the true scenery of each study population or in some studies possibly it is because of small number of study subjects. However though, the more convincing explanation of low malignancy level may be due to some pathologists incorporating the low grade malignancy into benign group. Neoplastic lesions such as polymorphous low grade adenocarcinoma (PLGA), basal cell adenocarcinoma (BCAC), epithelialmyoepithelial carcinoma (EMC) and malignant myoepithelioma (Table 6) with myxomatous or chondroid differentiation can simply be diagnosed as benign tumors due to absence of obvious microscopic malignant features.
Parotid and minor salivary gland tumors were much more likely to be malignant (54% in each) and submandibular tumors (30%) less likely to be malignant in this series. This finding parallels that of Malawi4 and the previous report from Uganda5. In Kenya series9 only palatal tumors were likely to be malignant. In the Western series the main site of malignancy is the submandibular gland followed by palate1–3,12,14,. This study concurs with Onyango et al9. account that, while the parotid gland is a less likely site for salivary gland tumor in African population, the tumors that occur in the gland have higher likelihood of being malignant than those from Western world. Parallel reflection should be applied to minor salivary gland tumors from black African population.
The Western reports shows that there is an equal sex distribution of malignant tumors, although the incidence for all salivary gland tumors shows a male to female ratio of 1.3:11–3,14. This is contrary to our observation since there was female predominance and more males had malignant than the females. Female predominance has been consistently reported from a number of African series4,5,7–11,15.
Whartin's tumors were not seen in this series. The rarity of this tumor in Africa has been noted previously4,5,8–10. In European and American series this tumor accounts between 5–20% of all salivary gland tumors 1–3,12,14. This has been attributed to the younger ages of Africans suffering from salivary gland tumors3. This reason is not convincing since the age range in African patients spans the average age range for patients with Whartin's tumor in Western series.
In this series the rarely and newly categorized tumors by WHO classification were not infrequently encountered (Table 4 and 5). Such recognition is important as it directs the management of such neoplasms. Since, not much is available in African literature, brief description of these new entities identified in this series is valuable.
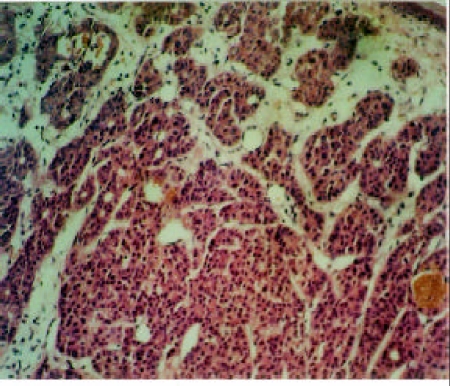
Basal cell adenocarcinoma (BCAC) is a rare tumor that has been reported to occur in the parotid and submandibular glands and very rarely in minor glands. It is considered to be a low-grade malignancy with a relatively high recurrence but good prognosis. BCAC and basal cell adenoma (BCA) share common clinical and histologic similarities (Figure 1). Both are composed of relatively uniform, monotonous, basaloid appearing cells arranged into solid, trabecular, tubular and membranous forms, and occasionally tumor nests may form pseudocystic spaces mimicking the cribriform pattern of adenoid cystic carcinoma. Nevertheless, BCAC and BCA differ from adenoid cyctic carcinoma in that they contain two types of cells, small round cells with scant cytoplasm and dark nuclei, and the larger polygonal to elongated cells with eosinophilic nucleus. Beside that, the frequent palisading of the nuclei along the epithelial-stromal interface, the lack of typical cribriform pattern, and the conspicuous hyaline lamina in membranous type may help to distinguish BCAC and BCA from ACC. Several histological hallmarks such as invasive outgrowth, perineural and vascular involvement, necrosis and some mitoses can distinguish BCAC from BCA. A careful search for these features would enable one to differentiate the two.
Figure 1.
Basal cell adenoma showing uniform basaloid cells arranged into solid, tubular and trabecular structures. Haematoxylin and Eosin (HE), X20
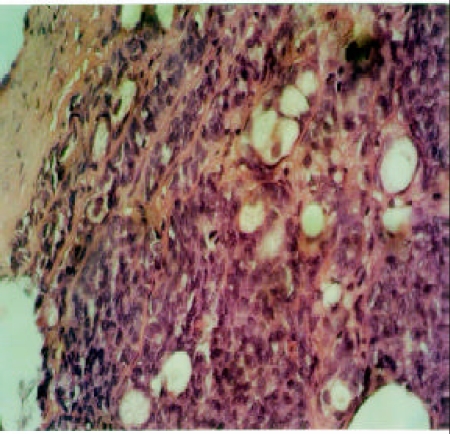
Polymorphous low grade adenocarcinoma (PLGA) is a low grade malignancy largely restricted in minor salivary glands, but cases from major salivary glands and seromucous glands of the nasopharynx have been reported. Microscopically, it consist of variable morphologic patterns in the same tumor namely, solid, tubular/trabecular and cribriform patterns. These feature may also be seen in ACC, however, fascicular areas, papillary structures and indian file alignment of tumor cells are confined to PLGA. In addition to that, uniform cells with vesicular nuclei and eosinophilic cytoplasm characterize PLGA. Pleomorphism is rarely seen, whereas mitoses can be identified (Figure 2).
Figure 2.
Plolymorphous low grade adenocarcinoma (PLGA) composed of uniform cells with vesicular nuclei and abundant eosinophilic cytoplasm. The cells form papillary or trabecular structures with Indian file alignment. Mitotic figure is seen at the centre. HE, X40.
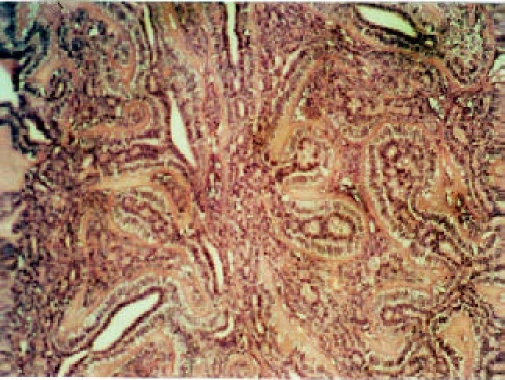
Epithelia-myoepithelial carcinoma (EMC) is a distinctive tumor that is relatively rare and accounts for only 1–2% of all primary salivary tumors. Whilst the parotid gland is the commonest location, EMC has been described in the submandibular gland, oral minor salivary glands, paranasal sinuses, bronchus, trachea, lacrimal glands, nasal cavity, external auditory canal and most recently in liver16. It was previously labelled as glycogen-rich or clear cell adenoma because of the clear component in the mistaken belief that it was a benign tumor of major salivary glands particulary the parotid. Cytologicaly, it is bland with rare mitoses. However, foci of necrosis and perineural and vascular invasion as seen in adenoid cystic carcinoma, are occasionally viewed in this tumor. Recurrence and metastasis are not uncommon. The pathgnomonic microscopic feature of EMC is the typical biphasic pattern of inner epithelial cells forming small ducts some of which may contain eosinophilic material, and the outer layer of myoepithelia cells that forms a circumferential clear cell collars around the ducts. The stroma is usually hyalinized with basement like bands that imparts nodularity of the tumor (Figure 3).
Figure 3.
Epithelial myoepithelial carcinoma characterized by glandular structures composed of inner epithelial cells and outer layer of myoepithelial cells with clear cytoplasm. The stroma is hyalinized. HE, X20.
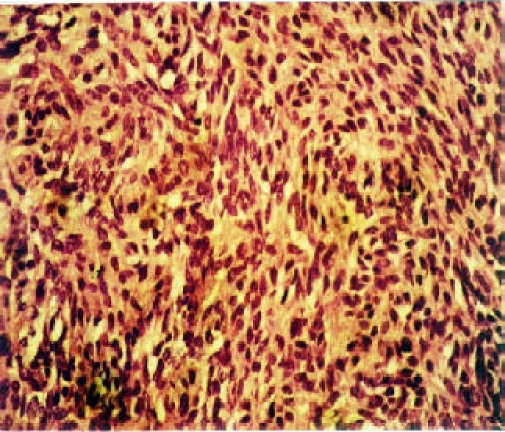
Benign and malignant myoepithelioma (the later also termed as myoepithelial carcinoma) are rare tumors accounting less than 1% of all salivary gland tumors. Malignant myoepithelioma is even more rare. Myoepitheliomas, both benign and malignant types are mainly located in the parotid gland. The age ranges from 48 years to 85 years with mean age of 62 years. Histologically, both tumors are comprised of one or mixture of epitheloid, spindle and/or plasmacytoid cell subtypes forming a solid, sheet-like growth pattern (Figure 3). Occasionally, small areas with chondromatous features and squamous differentiation may be observed in minority of cases, but glandular differentiation is inconspicuous. Malignant myoepithelioma may arise from a pre-existing pleomorphic adenoma, benign myoepithelioma or de-novo. Cellular pleomorphism of various degree, necrosis and invasion to adjacent tissue are often present in malignant myoepithelioma, but not evident in benign myoepithelioma. In few cases, anaplastic spindle-shaped cells intermingled with osteoclast-like giant cell may be seen in malignant myoepithelioma.
Conclusion
The findings of this study contribute significantly to the awareness of clinical and pathological features of salivary gland tumors in Uganda and black Africa at large. The study has employed the widely accepted WHO criteria and has been able to substantiate the clinical and pathological differences between African and non-African population. Since the reason for this difference has not been uncovered, further investigations specifically searching on the possible causes are greatly encouraged.
Figure 4.
Malignant myoepithelioma showing solid pattern composed of epitheloid and spindle shaped cell subtypes. Glandular differentiation is inconspiquous. HE, X40.
Acknowledgements
Sincere thanks and great appreciation are extended to the Dean of Faculty of Medicine Prof. N. Sewankambo who accessed my visit to the Department of Pathology, Makerere University. My great gratitudes to the Head, Department of Pathology, Makerere University and all members of the department for their invaluable support. My earnest acknowledgement to the Gender Dimension Committee of the University of Dar es Salaam, Tanzania, who funded the study.
References
- 1.Seifert G, Miehlke A, Haubrich J, Chilla R. Disease of the Salivary Glands: Pathology-Diagnosis-treatment-Facial Nerve Surg. New York: Georg Thieme Verlag Inc. Stuttgart; 1986. [Google Scholar]
- 2.Eveson JW, Cawson RA. Salivary gland tumors. A review of 2410 cases with particular reference to histological types, site, age and sex distribution. Journal of Pathology. 1985;146:51–58. doi: 10.1002/path.1711460106. [DOI] [PubMed] [Google Scholar]
- 3.Sharkey FE. Systematic evaluation of the World Health Organization Classification of salivary gland tumors: clinicopathological study of 366 cases. Amer J Clin Pathol. 1977;67:272–278. doi: 10.1093/ajcp/67.3.272. [DOI] [PubMed] [Google Scholar]
- 4.Thomas KM, Borgstein J. Salivary gland tumors in Malawi. Cancer. 1980;46:2328–2334. doi: 10.1002/1097-0142(19801115)46:10<2328::aid-cncr2820461036>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 5.Davies JNP, Dodge HD, Burkitt D. Salivary tumors in Uganda. Cancer. 1964;17:1310–1322. doi: 10.1002/1097-0142(196410)17:10<1310::aid-cncr2820171014>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 6.Seifert G, Sobin LH. Histological Typing of Salivary Gland Tumors. 2nd Edition. World Health Organization, Spring-Verlag; 1991. [Google Scholar]
- 7.Schulenburg CAR. Salivary Gland Tumors: A Report on 105 cases. South Afr Med J. 1954;8:910–914. [PubMed] [Google Scholar]
- 8.Kayembe MK, Kalenganyi MM. Salivary gland tumors in Congo (Zaire) Odontostomatol Trop. 2002;25(99):19–22. [PubMed] [Google Scholar]
- 9.Onyango JF, Awange DO, Muthamia JM, Muga BIO. Salivary Gland tumors in Kenya. East Africa Medical Journal. 1992;69(9):525–530. [PubMed] [Google Scholar]
- 10.Ezeanolue BC. Salivary gland neoplasms: a descriptive analysis of the pattern seen in Enugu. West Afr Med. 1999;18(3):179–182. [PubMed] [Google Scholar]
- 11.El-Gazayerli MM, Abdel-Aziz AS. Salivary gland tumors in Egypt and non-Western countries. British J of Cancer. 1964;18:649–654. doi: 10.1038/bjc.1964.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagler RM, Laufer D. Tumors of major and minor salivary glands: review of 25-year experience. Anticancer Res. 1997;17(1B):701–707. [PubMed] [Google Scholar]
- 13.Yu GY, Li ZL, Zhang Y. Diagnosis and treatment of epithelial salivary gland tumors in children and adolescents. British J of Oral and Maxillofacial Surgery. 2002;20(5):389–392. [PubMed] [Google Scholar]
- 14.Morgan MN, Mackenzie DH. Tumors of salivary glands. A review of 204 cases with 5-year follow-up. British J Surg. 1968;55(4):284–288. doi: 10.1002/bjs.1800550412. [DOI] [PubMed] [Google Scholar]
- 15.Ouoba K, Dao M, Sakande B, Kabre M, Cisse R, Ouedraogo I, Sanou A. Salivary gland tumors. Apropos of 48 cases. Dakar Med. 1998;43(1):60–64. [PubMed] [Google Scholar]
- 16.Chetty R. Intercalated duct hyperplasia: possible relationship to epithelial-myoepithelial carcinoma and hybrid tumors of salivary glands. Histopathology. 2000;37:260–263. doi: 10.1046/j.1365-2559.2000.00976.x. [DOI] [PubMed] [Google Scholar]