Abstract
In cooperatively breeding species, reproductive decisions and breeding roles may be influenced by environmental (food resources) or social factors (reproductive suppression of subordinates by dominants). Studies of glucocorticoid stress hormones in cooperatively breeding species suggest that breeding roles and hormone levels are related to the relative costs of dominance and subordination, which are driven primarily by social interactions. Few studies, however, have considered how environmental factors affect glucocorticoid levels and breeding roles in cooperative breeders, even though environmental stressors modulate seasonal glucocorticoid release and often influence breeding roles. I examined baseline and stress-induced levels of the glucocorticoid corticosterone (CORT) across 4 years in the plural breeding superb starling, Lamprotornis superbus, to determine whether (i) environmental factors (namely rainfall) directly influence breeding roles or (ii) environmental factors influence social interactions, which in turn drive breeding roles. Chronic baseline and maximal stress-induced CORT changed significantly across years as a function of pre-breeding rainfall, but dominant and subordinate individuals responded differently. Pre-breeding rainfall was also correlated directly with breeding roles. The results are most consistent with the hypothesis that environmental conditions influenced the relative costs of dominance and subordination, which in turn affected the degree and intensity of social interactions and ultimately reproductive decisions and breeding roles.
Keywords: glucocorticoids, corticosterone, allostasis, allostatic load, cooperative breeding, reproductive conflict
1. Introduction
Most cooperatively breeding animals live in kin-based family groups where more than two individuals care for young (Brown 1987). Many of the individuals who help to raise offspring forgo independent breeding, or if they do breed, they receive a smaller share of the reproduction than if they tried to breed independent of the group (Keller & Reeve 1994). This uneven sharing of reproduction (reproductive skew) often leads to conflict over reproductive opportunities and breeding roles between group members, otherwise known as reproductive conflict (Vehrencamp 1983). A variety of social and environmental factors influence levels of reproductive conflict and affect reproductive decisions and breeding roles. Ecological constraints such as limitation of suitable breeding sites (Walters 1990; Komdeur 1992) or access to resources on the natal territory (Dickinson & McGowan 2005; Baglione et al. 2006) can influence delayed dispersal (Emlen 1982), and access to food resources on the natal territory can affect breeding roles or the decision to breed or help (Clutton-Brock et al. 2001; Covas et al. 2004; Baglione et al. 2006). Moreover, dominant individuals may evict subordinates from natal territories (Stephens et al. 2005), or if subordinates are allowed to remain on the territory, dominants may reproductively suppress them through harassment (Curry 1988; Faulkes & Abbott 1997; Young et al. 2006).
Many studies have tried to identify causes of social suppression and social dominance in cooperatively breeding birds and mammals by measuring baseline levels of glucocorticoids, or stress hormones, in dominant and subordinate individuals (reviewed in Creel 2001; Goymann & Wingfield 2004). Glucocorticoids, such as corticosterone (CORT), are steroid hormones released from the adrenal glands in response to a variety of social and environmental stressors (Sapolsky et al. 2000). Whereas glucocorticoids are unlikely to cause different breeding roles themselves, they are likely to be indicative of the level of stress caused by different stressors. Although in most non-cooperative breeders, subordinates tend to have higher baseline glucocorticoid levels than dominants, initial comparative studies in cooperative breeders suggested that dominants tend to have equal or higher baseline glucocorticoid levels than subordinates (Creel 2001, 2005). This pattern was initially interpreted to mean that the maintenance of dominance rank may be more physiologically costly than subordination in cooperatively breeding species (Creel 2001, 2005; Sands & Creel 2004), rather than as support for the idea that reproductive suppression of helpers is related to glucocorticoids in cooperative breeders (Reyer et al. 1986; Schoech et al. 1991, 2004b, but see Young et al. 2006). However, across broad phylogenetic scales, patterns of glucocorticoids and breeding roles in cooperative breeders appear to be related to amounts of socially induced allostatic load or the costs of rank acquisition and maintenance for dominants relative to the costs of coping with aggressive interactions for subordinates (McEwen & Wingfield 2003; Goymann & Wingfield 2004). In other words, in species where it is more physiologically costly to be a dominant breeder (i.e. acquire and maintain dominance rank), dominants tend to have higher baseline glucocorticoids relative to subordinates, whereas in species where it is more costly to be a subordinate helper (i.e. the threat by dominants is greater), subordinates tend to have relatively higher glucocorticoid levels.
Although social factors may influence glucocorticoids and breeding roles (Goymann & Wingfield 2004), most studies of non-cooperatively breeding vertebrates have emphasized the importance of environmental factors on seasonal modulation of glucocorticoid release (Wingfield & Romero 2001; Romero 2002; Wingfield 2005). Since environmental factors (e.g. food or water abundance) influence glucocorticoid levels and the reproductive life histories of some cooperative breeders (Reynolds et al. 2003; Schoech & Bowman 2003; Schoech et al. 2004a), as well as reproductive decisions and breeding roles in many others (Clutton-Brock et al. 2001; Covas et al. 2004; Baglione et al. 2006), it seems important to examine simultaneously how both environmental and social stressors influence stress hormone levels and breeding roles in cooperatively breeding species. There are two hypotheses that may explain how social and environmental factors simultaneously influence breeding roles and reproductive decisions. First, social and environmental factors may each influence reproductive roles directly or via an interaction where the relative strength of each varies proportionally. According to this hypothesis, individuals may vary in how they respond to environmental factors in different reproductive contexts or life-history stages (Kitaysky et al. 1999; Goymann et al. 2001; Romero 2002). Such variation implies that individuals within a social group use different coping mechanisms in response to environmental stressors, and that some individuals may be more sensitive to unpredictable or fluctuating (i.e. stochastic) environments than others. Second, environmental factors may affect social factors, which in turn influence breeding roles. For example, studies of olive baboons, Papio anubis, have shown that during a severe drought, glucocorticoid levels in subordinates decreased as social interactions with dominants declined (Sapolsky 1986). According to this hypothesis, the relative costs of dominance and subordination can vary from year to year as environmental conditions change.
Here, I investigate how social and environmental factors are related to reproductive decisions and breeding roles in the cooperatively breeding superb starling, Lamprotornis superbus. Superb starlings are plural cooperative breeders that are endemic to the savannah bushland habitat of East Africa (Feare & Craig 1999; Fry et al. 2000), a temporally variable and unpredictable environment where rainfall varies greatly among years (Rubenstein 2006). I measured baseline and stress-induced CORT in birds captured in 4 successive years during the dry season immediately before the breeding period (hereafter, pre-breeding period). I compared levels in birds that became the social parents at a nest (breeders), those that helped provision nestling (helpers) and those that neither bred nor helped (non-breeders/non-helpers). I related CORT levels, standardized body mass (a measure of body condition) and breeding roles to pre-breeding rainfall to determine whether (i) environmental factors themselves correlate with CORT levels, standardized body mass and breeding roles or (ii) environmental factors influence social interactions, which in turn drive breeding roles. Since social suppression may be most evident during critical life-history stages (Young et al. 2006), I focused on the dry season immediately before breeding because this period influences breeding roles in many species of birds (Jacobs & Wingfield 2000), including some cooperative breeders (Emlen 1982; Canario et al. 2004). Additionally, although previous studies of glucocorticoids in avian cooperative breeders have examined singular breeders where reproductive conflict is generally low (Mays et al. 1991; Wingfield et al. 1991, 1992; Schoech et al. 1997), I specifically chose a plural cooperative breeder that lives in large complex family groups because the potential for reproductive conflict is high.
2. Material and methods
(a) Study system and species
Breeding activities of seven social groups were monitored from April 2001 to December 2005 at the Mpala Research Centre, Laikipia, Kenya (0°17′ N, 37°52′ E). One additional group was added in January 2002 and another was added in January 2003; both were monitored until December 2005. Groups of 10–35 (mean=21) individuals defended year-round territories and birds bred during both the long (March–May) and the short rains (November). Up to six breeding pairs per group (mean=3.5) bred during the long rains, and up to four breeding pairs per group (mean=1.7) bred during the short rains (Rubenstein 2006). Helpers, which aid in nestling provisioning, included both offspring and other first-order relatives (e.g. siblings, parents), as well as other group members that were less closely related, or even unrelated, to the breeding pair (Rubenstein 2006). Over 90% of nests had at least one helper, and the helpers had a positive effect on parental fitness by increasing the number of offspring fledged (Rubenstein 2006).
The main dry season (pre-breeding period) occurs between the short and the long rains and generally lasts from December to February. Daily rainfall data were collected during the study using a Hydrological Services TB3 Tipping Bucket Rain Gauge located at the Mpala Research Centre. The amount of rainfall that fell during the pre-breeding period was calculated as the sum of the daily rainfall during December, January and February of each year. This period represented the (i) three months with the lowest average cumulative monthly rainfall, (ii) three (of four) months where the variance in mean monthly rainfall was greater than the mean value, and (iii) three (of five) months when superb starlings did not initiate new clutches of eggs (Rubenstein 2006).
(b) Capture and blood sampling
Starlings were captured annually during the dry season using baited wire traps. Each individual was given a unique set of colour leg bands and a numbered metal leg ring. Birds were weighed to the nearest 0.5 g, and tarsus and wing cord length were measured to the nearest 0.1 and 0.5 cm, respectively. Body mass versus tarsus length for each individual was used in linear regression analyses to calculate the standardized body mass (i.e. residuals). Separate linear regressions were used for each year; each regression was positive and significant. Sex was unambiguously determined for all birds using PCR primers (Griffiths et al. 1998).
Blood samples for analysing baseline CORT were collected from either the jugular vein using a 10 cc syringe or the alar wing vein into capillary tubes within 3 min from the time of capture (mean=105 s; CORT does not tend to rise until 3 min after capture; Sapolsky et al. 2000; Romero 2004). After baseline samples were collected, birds were held in cloth bags and stress-induced CORT samples were taken at 10, 30, and 60 min after capture in 2003 and 2004, as well as at 90 min after capture in 2005. Stress series samples were not collected in 2002 because serial bleeding was not planned at the beginning of the study. The sample taken 60 min after capture was considered the maximal level of stress-induced CORT (see figure 2). Blood samples, including each stress series sample, were centrifuged at 4000 r.p.m. for 5 min in the field within 15 min of collection. Plasma was then stored on wet ice at 4°C for 1–4 h, at which time it was transferred to a −30°C freezer. All samples were exported on dry ice to the USA with permission from the Kenya Wildlife Service, the National Museums of Kenya Ornithology Department and the United States Department of Agriculture and held at −30°C until they were assayed. This work was approved by the Cornell Institutional Animal Care and Use Committee (01-27).
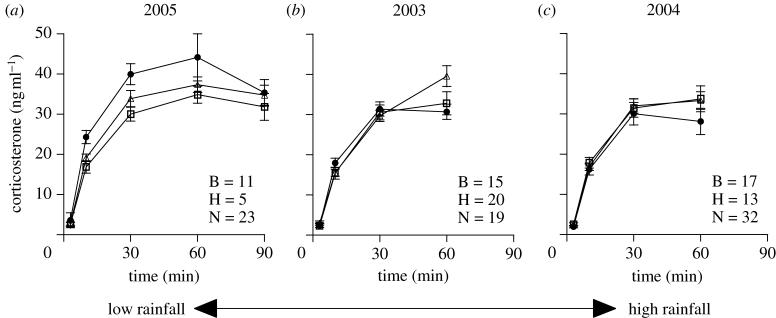
Figure 2.
Stress-induced corticosterone (CORT) in superb starlings measured over 3 years. Mean±s.e. stress-induced CORT in (a) 2005, (b) 2003 and (c) 2004 measured over a stress series from 3, 10, 30 and 60 min (and 90 min in 2005) after capture. Birds that became breeders (open squares), helpers (closed circles) and non-breeders/non-helpers (open triangles) had similarly shaped curves. Years are arranged according to the amount of rainfall received during the pre-breeding period (table 1). In 2005, the driest of the 3 years where stress-induced CORT was measured, birds that became helpers had significantly higher stress-induced CORT than those that became breeders and non-breeders/non-helpers. There were no differences in stress-induced CORT between birds that became breeders, helpers or non-breeders/non-helpers in the other 2 years. Sample sizes for birds that became breeders (B), helpers (H) and non-breeders/non-helpers (N) are indicated at the bottom of each panel.
The timing and duration of the capture period varied slightly among years with the onset of the rains, but capture effort was focused over the same life-history stage (pre-breeding period) each year. There was no effect of capture date on baseline CORT within any of the years (ANOVA: all p>0.21). Birds were captured in the mornings from 06.30 to 12.00 h and in the afternoons from 14.00 to 18.00 h. There was no relationship between capture time and baseline CORT either in the morning or in the afternoon (correlation: all p>0.12), and there were no differences in levels between birds caught in the morning and the afternoon (t-test: all p>0.40) for any of the years. Since there was no directional change in CORT for those individuals that were caught more than once each year, values were averaged for individuals caught repeatedly in a season. The number of birds captured each year varied: 92 unique birds were captured in 2002 (29 breeders, 19 helpers and 44 non-breeders/non-helpers); 63 unique birds in 2003 (15 breeders, 26 helpers and 22 non-breeders/non-helpers); 62 unique birds in 2004 (17 breeders, 13 helpers and 32 non-breeders/non-helpers); and 40 unique birds in 2005 (11 breeders, 5 helpers and 24 non-breeders/non-helpers). Although helping is male-biased in superb starlings (Rubenstein 2006), roughly equal numbers of male and female birds in each breeding role were captured in each year of this study. Sexes were combined for all analyses because (i) there were no differences in baseline or stress-induced CORT between males and females in any of the years (t-test: all p>0.21) and (ii) when ‘sex’ was entered as a covariate in all statistical models, neither sex nor any of the interactions with sex were significant predictors (ANOVA and ANCOVA: all p>0.51).
(c) Behavioural observations
Individual breeding roles were determined during the breeding season after the long rains began, weeks after the hormone samples were collected. Focal observations at nests (1–2 h) were conducted during the nest building and the incubation stages to identify the social breeders. Observers used spotting scopes and were hidden under a tree or behind a blind more than 30 m from the nest. Social mothers were identified as the female at a nest that incubated eggs and social fathers were defined as those that remained near the incubating female while the female was on the nest. After eggs hatched, focal observations (1–3 h) were used to identify helpers at the nest. Although most nests were observed multiple times after eggs hatched (range=1–9; mean=3.3), repeated observations were not always possible due to extremely high nest predation rates (nearly 75% of nests failed, with more than 90% due to nest predation; Rubenstein 2006).
The identity of all birds that came within ca 30 m of the nest and whether each bird brought food were recorded. Breeders were defined as the social parents of the nest, whereas helpers were defined as those individuals that brought food to a nest excluding parents. Although most breeders captured in this study were older than 2 years of age (70 out of 72, 97%), helpers were of all ages, but tended to be younger individuals; 41 out of 60 (68%) helpers captured in this study were 2 years of age or younger. All other group members that did not breed socially or help to provision young at the nest were considered non-breeders/non-helpers. This category comprised birds of all ages. Since some non-breeders/non-helpers could have been birds that (i) would have helped at a nest had it not been depredated during the egg stage or (ii) helped infrequently and were not observed during focal observations, the helper category represents the most conservative estimate.
(d) Aggressive interactions
Aggressive interactions in superb starlings were rare and occurred mainly in flight, which made it difficult to identify individual birds. However, ad hoc dominance interactions at feeding platforms baited with papaya were recorded during the pre-breeding period of 2002, the driest year of the study. The feeding platforms were located at baited-trapping locations at least twice a week at each site from January to March from 14.00 to 18.00 h. In the subsequent years of the study, birds were trapped in the afternoons at the same locations (also using papaya as bait), so there were no differences in the amount of food that birds had access to at any of the sites in any of the years. Birds were classified on a case-by-case basis as dominant, if they actively chased another individual, or subordinate, if they were chased by another bird. A total of 92 pairwise dominance interactions involving 70 unique individuals were observed. Birds that became breeders were more likely to be dominant, those that became helpers were more likely to be subordinate and those that became non-breeders/non-helpers were dominant about half of the time (chi-square test: χ2=12.62, n=184, p=0.0018). Specifically, birds that became breeders were dominant to those that became helpers (Fisher's exact test: n=21, p=0.0002), birds that became breeders were dominant to those that became non-breeders/non-helpers (Fisher's exact test: n=33, p<0.0001) and birds that became non-breeders/non-helpers were dominant to those that became helpers (Fisher's exact test: n=11, p=0.003).
(e) Hormone assays
Plasma levels of baseline CORT were determined by direct radioimmunoassay (RIA). Aliquots of 30–60 μl plasma were equilibrated with 2000 c.p.m. of 3H-corticosterone overnight at 4°C, extracted with dichloromethane, dried in a 40°C water bath under nitrogen gas and then re-dissolved in 550 μl PBSG buffer. After equilibrating with buffer overnight at 4°C, duplicate 200 μl fractions were taken for use in the RIA as described previously (CORT antibody, B3-163, Esoterix Endocrinology; Wingfield & Farner 1975). Additionally, 100 μl fractions were directly counted for the determination of recovery (mean recovery=82.1%). Two 400 μl aliquots of distilled water (blanks) and six 400 μl aliquots containing either 0.15, 0.25 or 0.50 ng non-radioactive CORT standards were taken through the whole assay procedure to estimate non-specific interference, assay accuracy and intra- and inter-assay variation. Blanks were always below the detection limits, which were set to the detection limit for each assay (range=0.41–0.66 ng ml−1). Thirty-four assays were conducted over the course of the study. The overall intra- and inter-assay variations were 10.8 and 10.4%, respectively.
(f) Statistics
Baseline CORT data were square root transformed to achieve normality; stress-induced CORT and standardized body mass did not require transformation. Two-way analysis of variance (ANOVA) with year, breeding role and their interaction was used to compare the mean baseline CORT values between birds that became breeders, helpers and non-breeders/non-helpers in each year. Independent contrasts on least square means were then used to compare the values within each year. An analysis of covariance (ANCOVA) was used to determine whether baseline CORT was related to pre-breeding rainfall for each reproductive class, and then separate linear regression analyses were conducted for birds that became helpers, those that became breeders and those that became non-breeders/non-helpers. Similar regression analyses were used to examine maximal stress-induced CORT. The t-tests were used to compare baseline and maximal stress-induced CORT in helpers during the driest year and the wettest year of the study for which samples of each type were collected. Similar ANOVA, ANCOVA and regression analyses were conducted on standardized body mass. A two-way ANOVA was used to determine whether differences in baseline CORT were related to differences in age or breeding role. Stress-induced levels of CORT at each stress series time period (10, 30, 60 or 90 min after capture) in each year (2003, 2004 and 2005) were analysed using two-way repeated measures ANOVA that included breeding role as a fixed effect, time period as a random effect to account for repeated measures during the stress series, and their interaction. Regressions analyses were used to determine whether the number of first-time breeders and the proportion of first-time breeders were related to pre-breeding rainfall.
3. Results
(a) Baseline corticosterone
Pre-breeding baseline CORT did not differ among years or among breeding roles (ANOVA: year: F3,243=0.28, p=0.84; breeding role: F2,243=0.56, p=0.57; interaction: F6,243=1.51, p=0.18). However, in one of the 4 years (2002), birds that became helpers during the breeding season had significantly higher CORT than those that became breeders and those that became non-breeders/non-helpers (p<0.01). To confirm that this pattern was driven by differences in breeding role and not age, I compared birds that became helpers and non-breeders/non-helpers, because these groups included individuals of all ages (0.5, 1, 2 or more than 2 years old); there were no breeders less than 2 years of age in 2002. Breeding role, but not age, was a significant predictor of baseline CORT (ANOVA: breeding role: F1,58=4.85, p=0.032; age: F3,58=1.73, p=0.17).
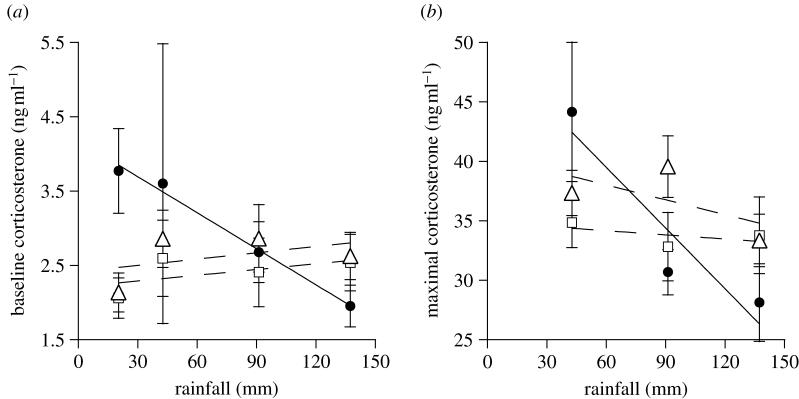
I determined whether year-to-year variation in baseline CORT was related to the amount of pre-breeding rainfall. Although there was no effect of pre-breeding rainfall or breeding role on baseline CORT, there was a significant interaction between the two factors (ANCOVA: rainfall: F1,243=0.47, p=0.50; breeding role: F2,243=1.37, p=0.26; interaction: F2,243=3.76, p=0.025). Birds that became helpers showed a significant negative relationship between pre-breeding rainfall and baseline CORT across years (regression: F1,61=4.48, p=0.038, R2=0.07; figure 1a), but there was no relationship in birds that became breeders (regression: F1,68=0.79, p=0.38, R2=0.01; figure 1a) or non-breeders/non-helpers (regression: F1,120=1.19, p=0.29, R2=0.01; figure 1a). Birds that became helpers had significantly higher baseline CORT in the driest year of the study than they did in the wettest year of the study (t-test: t=2.19, d.f.=30, p=0.037).
Figure 1.
Baseline and maximal stress-induced corticosterone (CORT) in superb starlings in relation to pre-breeding rainfall. Mean±s.e. pre-breeding (a) baseline and (b) maximal stress-induced CORT varied with pre-breeding rainfall in birds that became helpers (filled circles), but not in those that became breeders (open squares) or non-breeders/non-helpers (open triangles). Birds that became helpers had higher CORT in drier years than in wetter years. Sample sizes are indicated in the text.
(b) Stress-induced corticosterone
I examined stress-induced CORT in birds captured in 2003–2005. Although 2002 was the driest year of the study, 2005 was the driest of the 3 years where stress-induced CORT was analysed. In all the 3 years, CORT increased post-stress in all the groups (table 1; figure 2). Birds that became helpers had higher stress-induced CORT than those that became breeders and non-breeders/non-helpers in 2005 (the driest year in which stress series were conducted), but not in the other years (table 1; figure 2). Moreover, year-to-year variation in maximal stress-induced CORT levels (i.e. 60 min after capture) was related to the amount of pre-breeding rainfall, but only in birds that became helpers; birds that became helpers showed a significant negative relationship between pre-breeding rainfall and maximal stress-induced CORT across years (regression: F1,34=6.62, p=0.015, R2=0.16; figure 1b), but there was no relationship in birds that became breeders (regression: F1,41=0.036, p=0.85, R2=0.0009; figure 1b) or non-breeders/non-helpers (regression: F1,72=1.99, p=0.16, R2=0.03; figure 1b). Birds that became helpers had significantly higher maximal stress-induced CORT in the driest year of the study than they did in the wettest year of the study (t-test: t=2.18, d.f.=17, p=0.044).
Table 1.
Results of ANOVA models examining stress-induced corticosterone (CORT) in superb starlings. (Stress-induced CORT was measured at 10, 30 and 60 min after capture during the pre-breeding periods from 2003 to 2005 (and at 90 min after capture in 2005) in birds of each breeding role (breeders, helpers or non-breeders/non-helpers). There was a significant effect of sampling time period in each year, but there was only a significant effect of breeding role in 2005 (the driest of the 3 years) when birds that became helpers had significantly higher stress-induced CORT than those that became breeders or non-breeders/non-helpers. There was a significant interaction in 2003 such that birds that became non-breeders/non-helpers had significantly higher stress-induced CORT than those that became breeders or helpers at 60 min after capture. Bold values indicate significance at p<0.05.)
| year | rainfall (mm) | effect | d.f. | F | p |
|---|---|---|---|---|---|
| 2003 | 91.2 | time period | 2, 153 | 24.02 | 0.0058 |
| breeding role | 2, 153 | 0.30 | 0.76 | ||
| interaction | 6, 153 | 3.34 | 0.012 | ||
| 2004 | 137.4 | time period | 2, 177 | 109.02 | <0.0001 |
| breeding role | 2, 177 | 3.69 | 0.12 | ||
| interaction | 6, 177 | 0.33 | 0.86 | ||
| 2005 | 42.7 | time period | 3, 144 | 62.08 | <0.0001 |
| breeding role | 2, 144 | 20.99 | 0.002 | ||
| interaction | 6, 144 | 0.25 | 0.96 |
(c) Standardized body mass
If environmental factors directly influenced glucocorticoids and breeding roles in birds that became helpers, I would expect similar patterns in helpers between pre-breeding rainfall and CORT, as well as between pre-breeding rainfall and standardized body mass. Standardized body mass was not correlated with baseline (correlation: F1,252=0.74, p=0.39, r=0.054) or maximal stress-induced CORT (correlation: F1,151=0.54, p=0.46, r=0.06). There was no effect of pre-breeding rainfall on standardized body mass, but there was an effect of breeding role and a significant interaction (ANCOVA: rainfall: F1,250=2.41, p=0.12; breeding role: F2,250=6.81, p=0.0013; interaction: F2,250=3.26, p=0.04). Birds that became breeders had higher standardized body mass than those that became non-breeders/non-helpers (p=0.0004). Birds that became helpers (regression: F1,61=0.065, p=0.80, R2=0.001) or non-breeders/non-helpers (regression: F1,120=0.25, p=0.62, R2=0.002) did not show a relationship between standardized body mass and pre-breeding rainfall, but birds that became breeders showed a significant negative relationship (regression: F1,69=7.65, p=0.0073, R2=0.10).
(d) Breeding roles and reproductive decisions
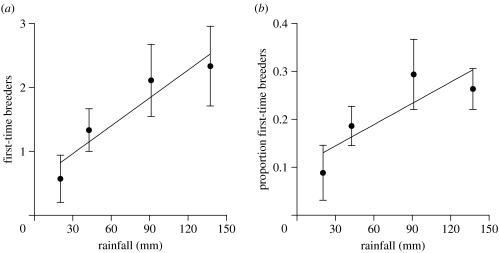
If pre-breeding rainfall influenced breeding roles, then fewer birds should breed for the first time following drier pre-breeding periods. There was a significant positive relationship between pre-breeding rainfall and the number of first-time breeders (regression: F1,32=6.52, p=0.016, R2=0.17; figure 3a), as well as the proportion of first-time breeders (regression: F1,32=5.16, p=0.03, R2=0.14; figure 3b) at each site the following breeding season.
Figure 3.
Superb starling breeding roles varied with pre-breeding rainfall. (a) The number of first-time breeders and (b) the proportion of first-time breeders increased with increasing amounts of pre-breeding rainfall. There were more, as well as a greater proportion of, first-time breeders following wetter pre-breeding periods, and fewer, as well as a smaller proportion of, first-time breeders following drier pre-breeding periods. Each point represents the mean±s.e. from all of the social groups in a given year.
4. Discussion
Environmental conditions significantly predicted glucocorticoid levels in superb starlings, but dominant (breeder) and subordinate (helper) individuals responded differently to among-year variation in rainfall. The amount of rainfall during the pre-breeding period was related to baseline and maximal stress-induced CORT in birds that became helpers, but not those that became breeders or non-breeders/non-helpers. Helpers had higher CORT in drier years and lower CORT in wetter years. These patterns could be attributed to differences in breeding role independent of age. Similar patterns in baseline and maximal stress-induced glucocorticoid levels are somewhat surprising, given that they can have different behavioural and physiological effects (Romero 2002, 2004). In fact, many studies of CORT in free-living birds often find non-significant differences in baseline levels among groups, but then significant differences in stress-induced levels in the predicted direction (e.g. Kitaysky et al. 1999; Romero & Wikelski 2002). Nonetheless, the results of this study suggest that in subordinate superb starlings, pre-breeding rainfall is related to both chronic baseline and maximal stress-induced CORT. Pre-breeding rainfall was also related to breeding roles directly; the number and proportion of first-time breeders in each group were higher following wetter years than following drier year. Although glucocorticoids are unlikely to cause different breeding roles themselves (i.e. measuring baseline or stress-induced CORT at single time points is unlikely to explain the causal mechanisms of reproductive behaviour), they are likely to be indicative of the level of stress caused by different social and environmental stressors.
Superb starlings live in a temporally variable environment where the amount of pre-breeding dry season rainfall varies greatly from year to year and influences food availability (Rubenstein 2006). Rainfall and food availability prior to the breeding season have been shown to drive reproductive decisions in many species of avian cooperative breeders. For example, rainfall influenced the number of birds that became breeders in white-throated bee-eaters, Merops bullockoides (Emlen 1982, 1990), and azure-winged magpies, Cyanopica cyanus (Canario et al. 2004). Furthermore, experimentally altered food availability influenced the timing of reproduction in Florida scrub-jays, Aphelocoma coerulescens (Schoech 1996; Schoech et al. 2004a), as well as which birds became breeders and helpers in sociable weavers, Philetarirus socius (Covas et al. 2004). In superb starlings, rainfall appears to be the environmental cue that drives reproductive decisions by influencing breeding roles, as well as the proportion of provisioning done by helpers (Rubenstein 2006). Glucocorticoids appear to be related to these decisions, at least in subordinate individuals. There are two probable explanations for these patterns: (i) subordinates were more physiologically sensitive to fluctuating and unpredictable environments than dominants or (ii) the relative costs associated with being dominant and subordinate varied from year to year with changing environmental conditions.
Nutrient availability and predictability in food resources have been directly linked to baseline CORT levels and reproductive decisions in cooperatively breeding Florida scrub-jays (Schoech et al. 2004a). However, although unpredictable food availability can cause an increase in baseline CORT in some birds (Pravosudov et al. 2001; Schoech et al. 2004a), its effect on baseline CORT in the European starling, Sturnus vulgaris (a non-cooperative breeder related to the superb starling), is less clear (Buchanan et al. 2003). To help determine whether pre-breeding rainfall had a direct effect on helper CORT in superb starlings, I examined a measure of body condition, standardized body mass, which was likely to be directly influenced by rainfall and food availability. There was no relationship between standardized body mass and pre-breeding rainfall in birds that became helpers. Surprisingly, standardized body mass showed a negative relationship with pre-breeding rainfall in birds that became breeders such that breeders weighed relatively more in drier years when pre-breeding conditions were harsher. Numerous studies in European starlings and other passerine birds have shown that body mass actually increases during periods of unpredictable food supply, probably due to reduced activity and metabolic expenditure and an increase in fat stores to endure the period of food shortage (Witter et al. 1995; Cuthill et al. 2000; Buchanan et al. 2003). Nonetheless, the lack of a consistent relationship between pre-breeding rainfall, CORT and standardized body mass in birds that became helpers suggests that rainfall and unpredictable food availability might not be directly related to CORT and breeding roles in superb starlings.
If subordinate helpers were less physiologically sensitive to fluctuating and unpredictable environments than were dominants, then glucocorticoid patterns and breeding roles may be related to among-year variation in the relative costs of dominance rank, which might be affected by the changing environmental conditions. According to reproductive skew theory, reproductive conflict in superb starlings would be expected to be highest during the driest years when breeding opportunities for subordinate individuals were lowest (Reeve & Shen 2006). Dominance interactions during the driest of the 4 years suggested that birds that became helpers were socially subordinate to those that became breeders or non-breeders/non-helpers. Thus, in the driest years, when helpers might be expected to have a greater effect on breeder fitness (Rubenstein 2006), the most subordinate birds in the group were likely to be harassed most, resulting in higher CORT and ultimately the adoption of helping roles. This may also explain why helpers did a greater proportion of the nest provisioning following drier pre-breeding periods (Rubenstein 2006). Group instability is known to influence glucocorticoid levels differently in dominant and subordinate individuals in group-living species (Sapolsky 1992, 1993; Bergman et al. 2005). Although superb starling groups were stable throughout the study, aggressive interactions (reproductive conflict) appeared to be higher in the drier years (D. Rubenstein 2002–2005, personal observation). Unfortunately, this could not be tested directly because natural cases of aggression and harassment occur in flight and the individuals involved cannot be easily identified. Although no similar studies have been done in birds, the patterns of aggression and glucocorticoids in superb starlings are opposite to those observed in olive baboons, where in drier years, aggressive interactions between dominants and subordinates decreased, and baseline glucocorticoid levels in subordinates declined (Sapolsky 1986). Further studies of species that exhibit high levels of reproductive conflict and that inhabit areas with high among-year variation in rainfall and food availability are needed to test the role that environmental factors play in influencing glucocorticoid levels, aggressive interactions and, ultimately, reproductive decisions in cooperatively breeding animals. Moreover, an examination of corticosteroid-binding globulins (CBGs) might help elucidate this issue, especially given that they may be related to seasonal patterns in corticosterone (Breuner & Orchinik 2002; Romero 2002) and that they are relevant to dominance interactions in other taxa (Alexander & Irvine 1998; Stefanski 2000).
In summary, the results of this study on superb starlings supports the predictions of allostatic load theory, which suggest that glucocorticoid concentrations are not monolithic and that both social and environmental factors influence allostatic load, or the relative costs of dominance and subordination, of breeders and helpers (McEwen & Wingfield 2003; Goymann & Wingfield 2004). Moreover, these results do not support the idea that subordinate helpers are generally less physiologically stressed than dominant breeders in cooperatively breeding species (Creel 2001, 2005; Sands & Creel 2004). Overall, the results are most consistent with the hypothesis that environmental factors may influence social interactions, which ultimately govern reproductive decisions and breeding roles in cooperatively breeding species. This relationship could explain why in many cooperative breeders, glucocorticoids are rarely directly related to reproductive suppression even though food availability influences reproductive decisions (e.g. Schoech et al. 1991, 2004a,b). As has been suggested from work in mammals (Young et al. 2006), relationships between glucocorticoid levels and breeding roles might only be observed during critical life-history stages such as the pre-breeding period when reproductive roles are being defined. My research further predicts that the link between breeding roles and glucocorticoids might only be observed in environmentally harsh years or when reproductive conflict is at its highest. Such patterns will be more likely to be observed in plural cooperatively breeding species and other systems where reproductive conflict is high, and reproductive skew is relatively low, rather than in singular breeders where conflict tends to be low and skew high.
Acknowledgments
J. Ekiru, F. Lomojo, G. Rana, J. Ronjore and W. Watetu helped in the field. M. Hau and M. Wikelski provided guidance in the laboratory. This manuscript was improved by comments from E. Adkins-Regan, S. Emlen, J. Lee, I. Lovette, L. Martin, K. McGraw, D. I. Rubenstein, P. Sherman, M. Wikelski and three anonymous reviewers. I thank the Kenyan Ministry of Education, Science and Technology, the National Museums of Kenya Ornithology Department, the Kenya Wildlife Service and the Mpala Research Centre for enabling this work. This research was supported by fellowships from the Howard Hughes Medical Institute, the Smithsonian Institution and the Cornell University College of Agriculture and Life Sciences, as well as by grants from the National Science Foundation (IBN-407713), the American Museum of Natural History Chapman Fund, the American Ornithologists' Union, the Wilson Ornithological Society, the Society for Integrative and Comparative Biology, the Animal Behaviour Society, the Andrew W. Mellon Foundation, the Harvard Travellers Club, the Society of Sigma Xi, Cornell University, the Cornell Laboratory of Ornithology Benning Fund and Cornell Sigma Xi.
References
- Alexander S.L, Irvine C.H.G. The effect of social stress on adrenal axis activity in horses: the importance of monitoring corticosteroid-binding globulin capacity. J. Endocrinol. 1998;157:425–432. doi: 10.1677/joe.0.1570425. doi:10.1677/joe.0.1570425 [DOI] [PubMed] [Google Scholar]
- Baglione V, Canestrari D, Marcos J.M, Ekman J. Experimentally increased food resources in the natal territory promote offspring philopatry and helping in cooperatively breeding carrion crows. Proc. R. Soc. B. 2006;273:1529–1535. doi: 10.1098/rspb.2006.3481. doi:10.1098/rspb.2006.3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman T.J, Beehner J.C, Cheney D.L, Seyfarth R.M, Whitten P.L. Correlates of stress in free-ranging male chacma baboons, Papio hamadryas ursinus. Anim. Behav. 2005;70:703–713. doi:10.1016/j.anbehav.2004.12.017 [Google Scholar]
- Breuner C.W, Orchinik M. Plasma binding proteins as mediators of corticosteroid action in vertebrates. J. Endocrinol. 2002;175:99–112. doi: 10.1677/joe.0.1750099. doi:10.1677/joe.0.1750099 [DOI] [PubMed] [Google Scholar]
- Brown J.L. Princeton University Press; Princeton, NJ: 1987. Helping and communal breeding in birds: ecology and evolution. [Google Scholar]
- Buchanan K.L, Spencer K.A, Goldsmith A.R, Catchpole C.K. Song as an honest signal of past developmental stress in the European starling (Sturnus vulgaris) Proc. R. Soc. B. 2003;270:1149–1156. doi: 10.1098/rspb.2003.2330. doi:10.1098/rspb.2003.2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canario F, Matos S, Soler M. Environmental constraints and cooperative breeding in the azure-winged magpie. Condor. 2004;106:608–617. doi:10.1650/7454 [Google Scholar]
- Clutton-Brock T.H, Russell A.F, Sharpe L.L, Brotherton P.N.M, McIlrath G.M, White S, Cameron E.Z. Effects of helpers on juvenile development and survival in meerkats. Science. 2001;293:2446–2449. doi: 10.1126/science.1061274. doi:10.1126/science.1061274 [DOI] [PubMed] [Google Scholar]
- Covas R, Doutrelant C, du Plessis M.A. Experimental evidence of a link between breeding condition and the decision to breed or help in a colonial cooperative bird. Proc. R. Soc. B. 2004;271:827–832. doi: 10.1098/rspb.2003.2652. doi:10.1098/rspb.2003.2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creel S. Social dominance and stress hormones. Trends Ecol. Evol. 2001;16:491–497. doi:10.1016/S0169-5347(01)02227-3 [Google Scholar]
- Creel S. Dominance, aggression, and glucocorticoid levels in social carnivores. J. Mammal. 2005;86:255–264. doi:10.1644/BHE-002.1 [Google Scholar]
- Curry R.L. Group structure, within-group conflict and reproductive tactics in cooperatively breeding Galapagos mockingbirds, Nesomimus parvulus. Anim. Behav. 1988;36:1708–1728. doi:10.1016/S0003-3472(88)80111-8 [Google Scholar]
- Cuthill I.C, Maddocks S.A, Weall C.V, Jones E.K.M. Body mass regulation in response to changes in feeding predictability and overnight energy expenditure. Behav. Ecol. 2000;11:189–195. doi:10.1093/beheco/11.2.189 [Google Scholar]
- Dickinson J.L, McGowan A. Winter resource wealth drives delayed dispersal and family-group living in western bluebirds. Proc. R. Soc. B. 2005;272:2423–2428. doi: 10.1098/rspb.2005.3269. doi:10.1098/rspb.2005.3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emlen S.T. The evolution of helping. 1. An ecological constraints model. Am. Nat. 1982;119:29–39. doi:10.1086/283888 [Google Scholar]
- Emlen S.T. White-fronted bee-eaters: helping in a colonially nesting species. In: Stacey P.B, Koenig W.D, editors. Cooperative breeding in birds: long-term studies of ecology and behavior. Cambridge University Press; Cambridge, UK: 1990. pp. 489–526. [Google Scholar]
- Faulkes C.G, Abbott D.H. The physiology of reproductive dictatorship: regulation of male and female reproduction by a single breeding female in colonies of naked mole-rats. In: Solomon N.G, French J.A, editors. Cooperative breeding in mammals. Cambridge University Press; New York, NY: 1997. pp. 302–334. [Google Scholar]
- Feare C, Craig A. Princeton University Press; Princeton, NJ: 1999. Starlings and mynas. [Google Scholar]
- Fry C.H, Keith S, Urban E.K. Academic Press; San Diego, CA: 2000. The birds of Africa. [Google Scholar]
- Goymann W, Wingfield J.C. Allostatic load, social status and stress hormones: the costs of social status matter. Anim. Behav. 2004;67:591–602. doi:10.1016/j.anbehav.2003.08.007 [Google Scholar]
- Goymann W, East M.L, Wachter B, Honer O.P, Mostly E, Van't Hof T.J, Hofer H. Social, state-dependent and environmental modulation of faecal corticosteroid levels in free-ranging female spotted hyenas. Proc. R. Soc. B. 2001;268:2453–2459. doi: 10.1098/rspb.2001.1828. doi:10.1098/rspb.2001.1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R, Double M.C, Orr K, Dawson R.J.G. A DNA test to sex most birds. Mol. Ecol. 1998;7:1071–1075. doi: 10.1046/j.1365-294x.1998.00389.x. doi:10.1046/j.1365-294x.1998.00389.x [DOI] [PubMed] [Google Scholar]
- Jacobs J.D, Wingfield J.C. Endocrine control of life-cycle stages: a constraint on response to the environment? Condor. 2000;102:35–51. doi:10.1650/0010-5422(2000)102[0035:ECOLCS]2.0.CO;2 [Google Scholar]
- Keller L, Reeve H.K. Partitioning of reproduction in animal societies. Trends Ecol. Evol. 1994;9:98–102. doi: 10.1016/0169-5347(94)90204-6. doi:10.1016/0169-5347(94)90204-6 [DOI] [PubMed] [Google Scholar]
- Kitaysky A.S, Wingfield J.C, Piatt J.F. Dynamics of food availability, body condition and physiological stress response in black-legged kittiwakes. Funct. Ecol. 1999;13:577–584. doi:10.1046/j.1365-2435.1999.00352.x [Google Scholar]
- Komdeur J. Importance of habitat saturation and territory quality for evolution of cooperative breeding in the Seychelles warbler. Nature. 1992;358:493–495. doi:10.1038/358493a0 [Google Scholar]
- Mays N.A, Vleck C.M, Dawson J. Plasma luteinizing hormone, steroid hormones, behavioral role, and nest stage in cooperatively breeding Harris hawks (Parabuteo unicinctus) Auk. 1991;108:619–637. [Google Scholar]
- McEwen B.S, Wingfield J. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. doi:10.1016/S0018-506X(02)00024-7 [DOI] [PubMed] [Google Scholar]
- Pravosudov V.V, Kitaysky A.S, Wingfield J.C, Clayton N.S. Long-term unpredictable foraging conditions and physiological stress response in mountain chickadees (Poecile gambeli) Gen. Comp. Endrocrinol. 2001;123:324–331. doi: 10.1006/gcen.2001.7684. doi:10.1006/gcen.2001.7684 [DOI] [PubMed] [Google Scholar]
- Reeve H.K, Shen S.-F. A missing model in reproductive skew theory: the bordered tug-of-war. Proc. Natl Acad. Sci. USA. 2006;103:8430–8434. doi: 10.1073/pnas.0603005103. doi:10.1073/pnas.0603005103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyer H.U, Dittami J.P, Hall M.R. Avian helpers at the nest: are they psychologically castrated? Ethology. 1986;71:216–228. [Google Scholar]
- Reynolds S.J, Schoech S.J, Bowman R. Nutritional quality of pre-breeding diet influences breeding performance of the Florida scrub-jay. Oecology. 2003;134:308–316. doi: 10.1007/s00442-002-1126-y. doi:10.1007/s00442-002-1126-y [DOI] [PubMed] [Google Scholar]
- Romero L.M. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. Endrocrinol. 2002;128:1–24. doi: 10.1016/s0016-6480(02)00064-3. doi:10.1016/S0016-6480(02)00064-3 [DOI] [PubMed] [Google Scholar]
- Romero L.M. Physiological stress in ecology: lessons from biomedical research. Trends Ecol. Evol. 2004;19:249–255. doi: 10.1016/j.tree.2004.03.008. doi:10.1016/j.tree.2004.03.008 [DOI] [PubMed] [Google Scholar]
- Romero L.M, Wikelski M. Exposure to tourism reduced stress-induced corticosterone levels in Galapagos marine iguanas. Biol. Conserv. 2002;108:371–374. doi:10.1016/S0006-3207(02)00128-3 [Google Scholar]
- Rubenstein, D. R. 2006 The evolution of the social and mating systems of the plural cooperatively breeding superb starling, Lamprotornis superbus Ph.D. dissertation. Ithaca, NY: Cornell University.
- Sands J, Creel S. Social dominance, aggression and faecal glucocorticoid levels in a wild population of wolves, Canis lupus. Anim. Behav. 2004;67:387–396. doi:10.1016/j.anbehav.2003.03.019 [Google Scholar]
- Sapolsky R.M. Endocrine and behavioral correlates of drought in the wild baboon. Am. J. Primatol. 1986;11:217–228. doi: 10.1002/ajp.1350110303. doi:10.1002/ajp.1350110303 [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M. Cortisol concentrations and the social significance of rank instability among wild baboons. Psychoneuroendocrinology. 1992;17:701–709. doi: 10.1016/0306-4530(92)90029-7. doi:10.1016/0306-4530(92)90029-7 [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M. The physiology of dominance in stable versus unstable hierarchies. In: Mason W.A, Mendoza S.P, editors. Primate social conflict. State University of New York Press; Albany, NY: 1993. pp. 171–204. [Google Scholar]
- Sapolsky R.M, Romero L.M, Munck A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. doi:10.1210/er.21.1.55 [DOI] [PubMed] [Google Scholar]
- Schoech S.J. The effect of supplemental food on body condition and the timing of reproduction in a cooperative breeder, the Florida scrub-jay (Aphelocoma coerulescens) Condor. 1996;98:234–244. [Google Scholar]
- Schoech S.J, Bowman R. Does differential access to protein influence differences in timing of breeding of Florida Scrub-Jays (Aphelocoma coerulescens) in suburban and wildlands habitats? Auk. 2003;120:1114–1127. doi:10.1642/0004-8038(2003)120[1114:DDATPI]2.0.CO;2 [Google Scholar]
- Schoech S.J, Mumme R.L, Moore M.C. Reproductive endocrinology and mechanisms of breeding inhibition in cooperatively breeding Florida scrub jays (Aphelocoma c. coerulescens) Condor. 1991;93:354–364. [Google Scholar]
- Schoech S.J, Mumme R.L, Wingfield J.C. Corticosterone, reproductive status, and body mass in a cooperative breeder, the Florida scrub jay (Aphelocoma coerulescens) Physiol. Zool. 1997;70:68–73. doi: 10.1086/639545. [DOI] [PubMed] [Google Scholar]
- Schoech S.J, Bowman R, Reynolds S.J. Food supplementation and possible mechanisms underlying early breeding in the Florida scrub-jay (Aphelocoma coerulescens) Horm. Behav. 2004a;46:565–573. doi: 10.1016/j.yhbeh.2004.06.005. doi:10.1016/j.yhbeh.2004.06.005 [DOI] [PubMed] [Google Scholar]
- Schoech S.J, Reynolds S.J, Boughton R.K. Endocrinology. In: Koenig W.D, Dickinson J.L, editors. Ecology and evolution of cooperative breeding in birds. Cambridge University Press; Cambridge, UK: 2004b. pp. 128–141. [Google Scholar]
- Stefanski V. Social stress in laboratory rats: hormonal responses and immune cell distribution. Psychoneuroendocrinology. 2000;25:389–406. doi: 10.1016/s0306-4530(99)00066-9. doi:10.1016/S0306-4530(99)00066-9 [DOI] [PubMed] [Google Scholar]
- Stephens P.A, Russell A.F, Young A.J, Sutherland W.J, Clutton-Brock T.H. Dispersal, eviction, and conflict in meerkats (Suricata suricatta): an evolutionarily stable strategy model. Am. Nat. 2005;165:120–135. doi: 10.1086/426597. doi:10.1086/426597 [DOI] [PubMed] [Google Scholar]
- Vehrencamp S.L. Optimal degree of skew in cooperative societies. Am. Zool. 1983;23:327–335. [Google Scholar]
- Walters J.R. Red-cockaded woodpeckers: a ‘primitive’ cooperative breeder. In: Stacey P.B, Koenig W.D, editors. Cooperative breeding in birds: long-term studies of ecology and behavior. Cambridge University Press; Cambridge, UK: 1990. pp. 67–102. [Google Scholar]
- Wingfield J.C. The concept of allostasis: coping with a capricious environment. J. Mamm. 2005;86:248–254. doi:10.1644/BHE-004.1 [Google Scholar]
- Wingfield J.C, Farner D.S. Determination of 5 steroids in avian plasma by radioimmunoassay and competitive protein binding. Steroids. 1975;26:311–327. doi: 10.1016/0039-128x(75)90077-x. doi:10.1016/0039-128X(75)90077-X [DOI] [PubMed] [Google Scholar]
- Wingfield J.C, Romero L.M. Adrenocortical responses to stress and their modulation in free-living vertebrates. In: McEwen B.S, Goodman H.M, editors. Handbook of physiology; section 7: the endocrine system. Coping with the environment: neural and endocrine mechanisms.(IV) Oxford University Press; New York, NY: 2001. pp. 211–234. [Google Scholar]
- Wingfield J.C, Hegner R.E, Lewis D.M. Circulating levels of luteinizing hormone and steroid hormones in relation to social status in the cooperatively breeding white-browed sparrow weaver, Plocepasser mahali. J. Zool. 1991;225:43–58. [Google Scholar]
- Wingfield J.C, Hegner R.E, Lewis D.M. Hormonal responses to removal of a breeding male in the cooperatively breeding white-browed sparrow weaver, Plocepasser mahali. Horm. Behav. 1992;26:145–155. doi: 10.1016/0018-506x(92)90038-w. doi:10.1016/0018-506X(92)90038-W [DOI] [PubMed] [Google Scholar]
- Witter M.S, Swaddle J.P, Cuthill I.C. Periodic food availability and strategic regulation of body mass in the European Starling, Sturnus vulgaris. Funct. Ecol. 1995;9:568–574. doi:10.2307/2390146 [Google Scholar]
- Young A.J, Carlson A.A, Monfort S.L, Russell A.F, Bennett N.C, Clutton-Brock T.H. Stress and the suppression of subordinate reproduction in cooperatively breeding meerkats. Proc. Natl Acad. Sci. USA. 2006;103:12 005–12 010. doi: 10.1073/pnas.0510038103. doi:10.1073/pnas.0510038103 [DOI] [PMC free article] [PubMed] [Google Scholar]