Scheme 6.
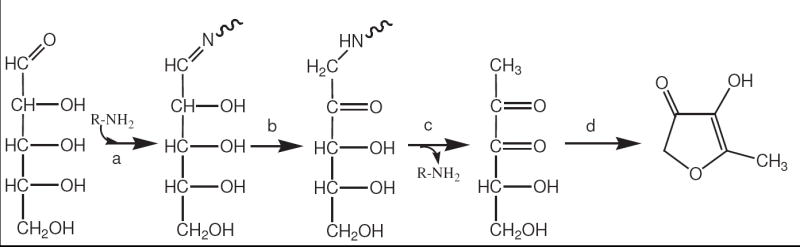
A proposed sequence of reactions between d-ribose and NAcLys leading to a m/z 115 [M + H+] product. The reaction is initiated (step a) by an attachment of the amine to form a Schiff base, followed by Amadori rearrangement (step b) and hydrolysis of the amine to yield a 1-deoxy-2,3-dicarbonyl compound (step c). The dicarbonyl compound can spontaneously rearrange to HMF by a previously reported mechanism.10
