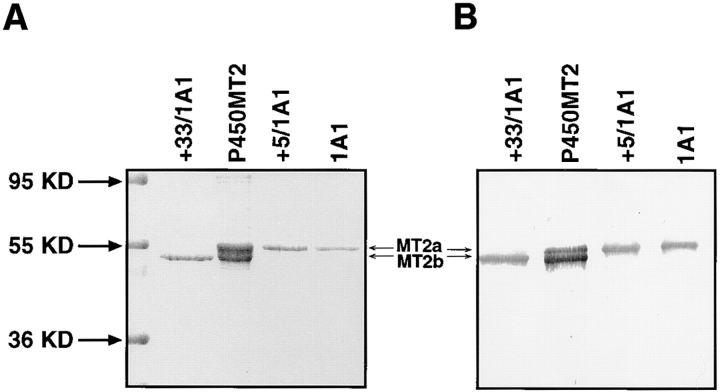
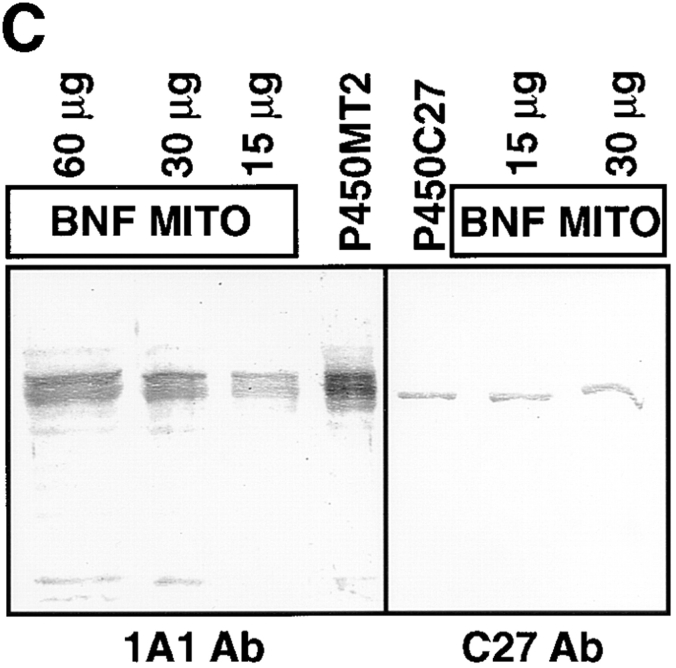
Figure 1.
Electrophoretic resolution of the two forms of mitochondrial P450MT2. P450s from BNF-induced rat liver mitochondria and microsomes and bacterially expressed +5/ 1A1 and +33/1A1 were purified, and ∼1–2 μg of protein sample in each case was resolved by electrophoresis on a 14–16% gradient SDS-polyacrylamide gel. (A) Coomassie Blue stained pattern. (B) A companion gel was subjected to Western blot analysis using 20 μg/ml of affinity-purified P4501A1 antibody. (C) The purified P450MT2 was compared with antibody-reactive proteins from BNF-induced liver mitochondria. Indicated amounts of mitochondria from BNF-induced liver and 2 μg of P450MT2 were subjected to electrophoretic resolution and Western blot analysis using either affinity-purified P4501A1 antibody or mAb against P450c27 (Addya et al., 1991), as indicated.