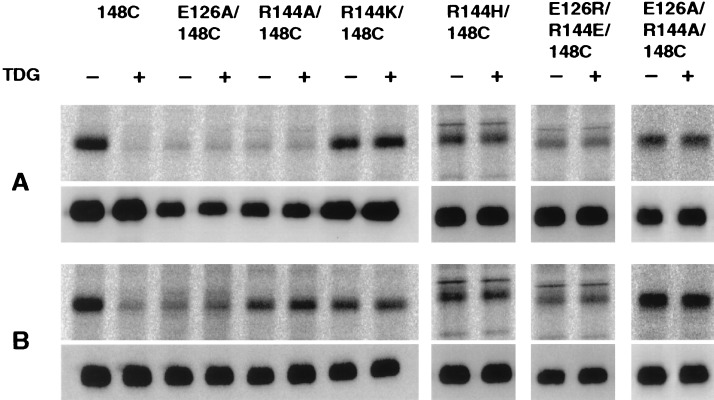
Figure 3.
Reactivity of single-Cys permease mutants with [1-14C]NEM and the effect of TDG. Membranes [0.2–0.4 mg of protein in 50 μl of 100 mM KPi (pH 7.5)/10 mM MgSO4] prepared from E. coli T184 transformed with pKR35/lacY-CXB encoding single-Cys mutants E126A/C148, R144A/C148, R144K/C148, R144H/C148, E126R/R144E/C148, or E126A/R144A/C148, as indicated, were incubated with [1-14C]NEM (40 mCi/mmol; 0.4 mM final concentration) for 10 min (A) or 30 min (B) in the absence or presence of 10 mM TDG at 25°C. The reactions were terminated by the addition of DTT to a final concentration of 15 mM, and biotinylated permease was solubilized and purified as described (14). Aliquots containing 5 μg of protein were separated by sodium dodecyl sulfate/12% PAGE, and the labeled protein was visualized by autoradiography (Upper, A and B). A fraction of the eluted protein (0.5 μg) was analyzed by Western blotting with anti-C-terminal antibody (Lower, A and B).