Figure 10.
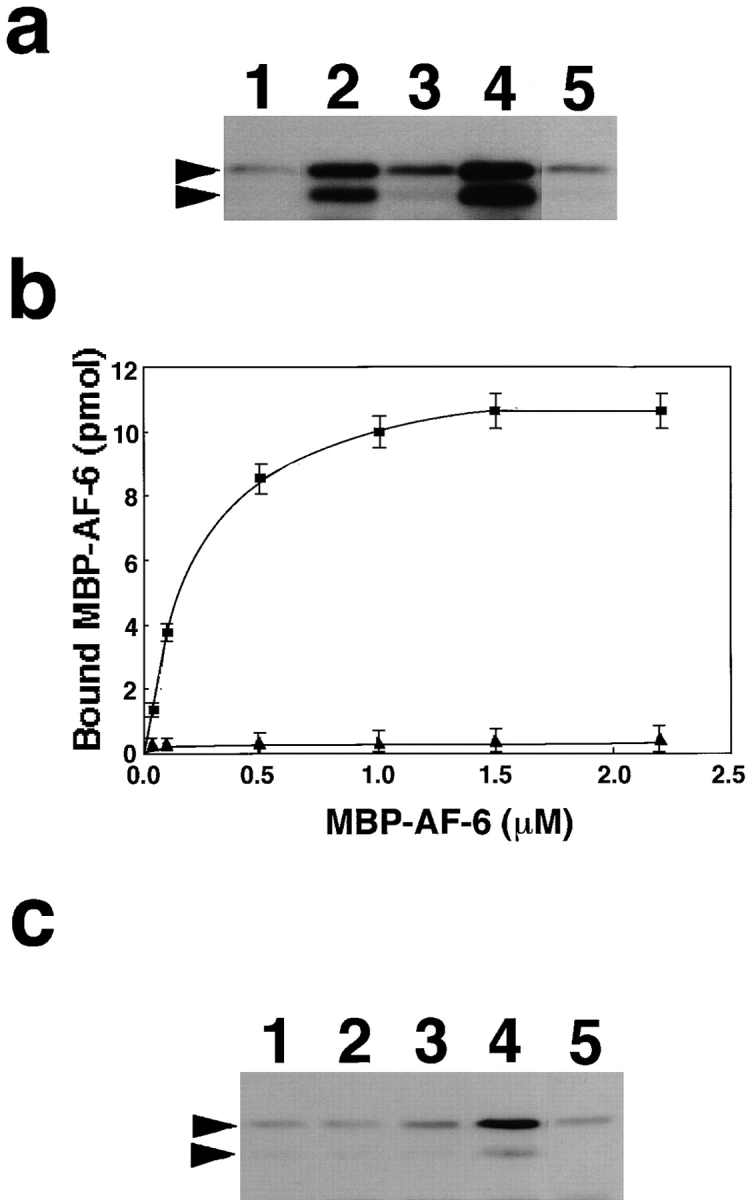
Dissociation of the Ras-interacting domain of AF-6 from ZO-1 by activated Ras. (a) Interaction of MBP-AF-6 (36– 206 amino acids) with ZO-1. Crude lysates of E. coli expressing MBP-AF-6 (36–206 amino acids) were loaded onto affinity columns immobilized with GST (lane 1), GST-ZO-1 (lane 2), GDP/ GST–Ha-Ras (lane 3), GTPγS/GST–Ha-Ras (lane 4), and GST-CD44 (lane 5). The interacting proteins were eluted with GST fusion proteins by the addition of glutathione. The eluates were subjected to SDS-PAGE and followed by immunoblot analysis with anti-MBP antibody. The arrowheads denote the positions of MBP-AF-6 (36–206 amino acids). (b) The kinetic study on the binding of MBP-AF-6 (36–206 amino acids) to GST-ZO-1. E. coli lysates containing various concentrations of MBP-AF-6 (36–206 amino acids) were loaded onto the GST-ZO-1 and GST-CD44 affinity columns (0.1 nmol). The proteins bound to GST-ZO-1 columns were eluted with GST-ZO-1 by the addition of glutathione. The eluates were subjected to SDS-PAGE and followed by immunoblot analysis with anti-MBP antibody. The immunodetected MBP-AF-6 were visualized and estimated with a densitograph. The values shown are means ± SEM of triplicate experiments. ▪, with GST-ZO-1; ▴, with GST-CD44. (c) Dissociation of MBP-AF-6 (36–206 amino acids) from ZO-1 by activated Ras. Crude lysates of E. coli expressing MBP-AF-6 (36–206 amino acids) were loaded onto affinity columns immobilized with GST-ZO-1. The proteins bound to the GST-ZO-1 columns were eluted by the addition of buffer containing GDP alone (lane 1), GTPγS alone (lane 2), GDP/Ras (lane 3), GTPγS/Ras (lane 4), and GTPγS/Rac (lane 5). The eluates were subjected to SDS-PAGE and followed by immunoblot analysis with anti-MBP antibody. The arrowhead denotes the position of MBP-AF-6 (36–206 amino acids). The results shown are representative of three independent experiments.
