Figure 4.
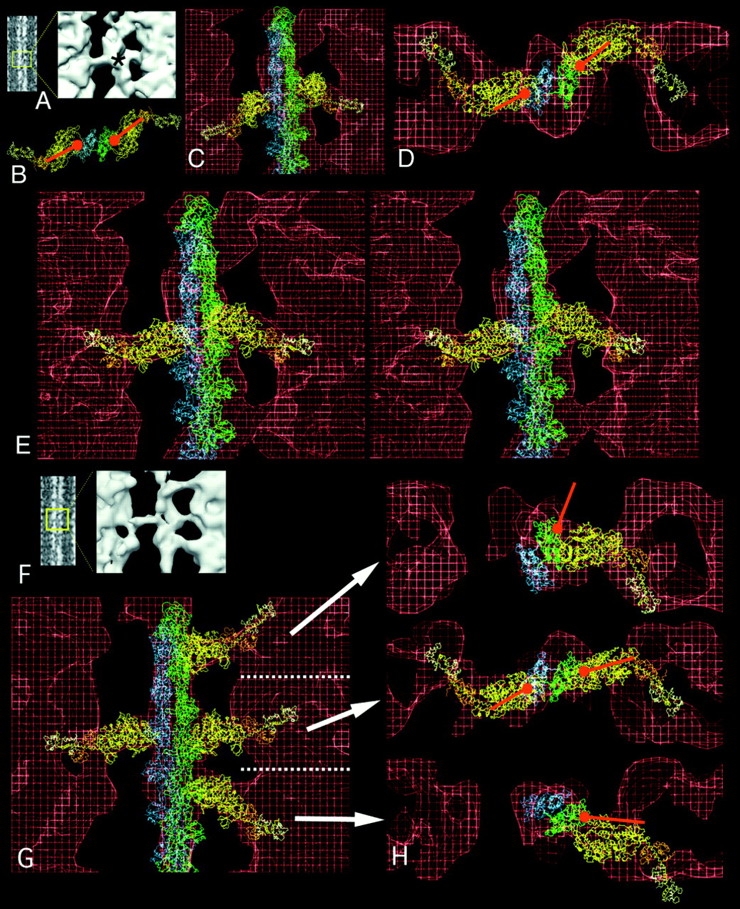
Fittings of the acto-S1 atomic model into target zone and nontarget zone crossbridges of IFM in glycol-PNP. (A) Projection image in which the particular motif is boxed and enlarged in the surface view. The asterisk marks the level of the actin target zone. B is a view down the filament axis of the rigor atomic model. The orientation of the red ball and stick identify the binding site and azimuthal angle of rigor S1. (C) Unmodified rigor acto-S1 model superimposed onto target zone crossbridges shows that the 45° angle of rigor is not matched by the 90° angle of glycol-PNP crossbridges. (D) Transverse view of target zone crossbridge seen in E, fitted with S1 whose axial angle has been changed to 90°. (E) Stereo longitudinal view (ocular divergence) of target zone bridges showing the good fit achieved when the axial angle of S1 is pivoted to 90°. In longitudinal view, the motor domain of the S1 (C–E, yellow) and the regulatory domain (orange and white) fit into the crossbridge envelope (red). (F) Projection and surface image of a region containing two target zone and two nontarget zone crossbridges. (G) Acto-S1 model superimposed on the reconstruction. The orientation of S1 has been changed to align with each crossbridge envelope. (H) Transverse view of the same fitting of each of the three crossbridge levels in G, showing that while the target zone bridges have similar azimuthal orientation as rigor S1 and bind to the same place on actin, the nontarget bridges do not. In particular, to align S1 with the upper nontarget bridge envelope required moving the actin–S1 interface to a different part of actin.
