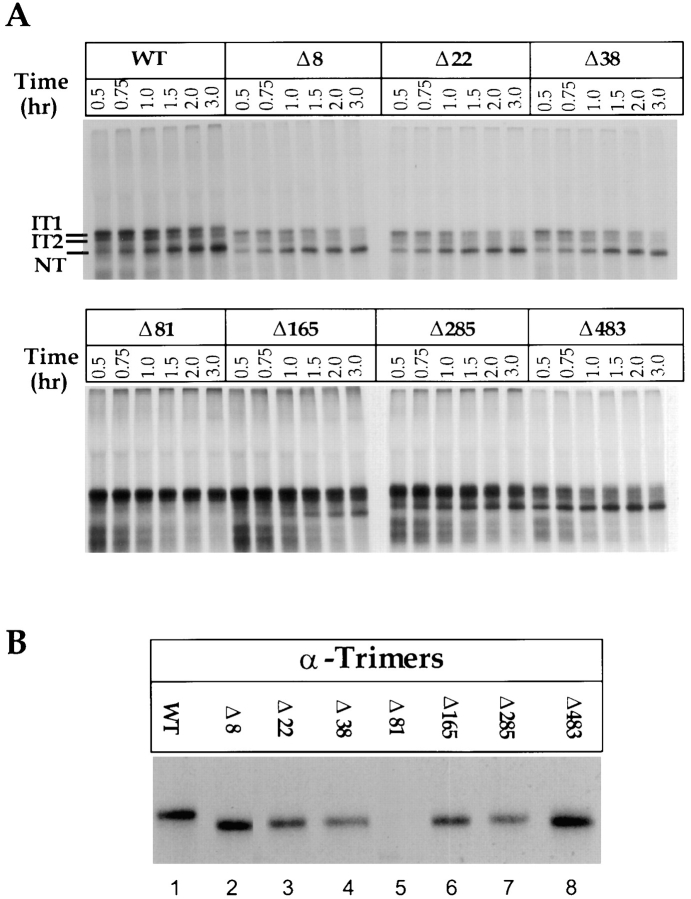
Figure 4.
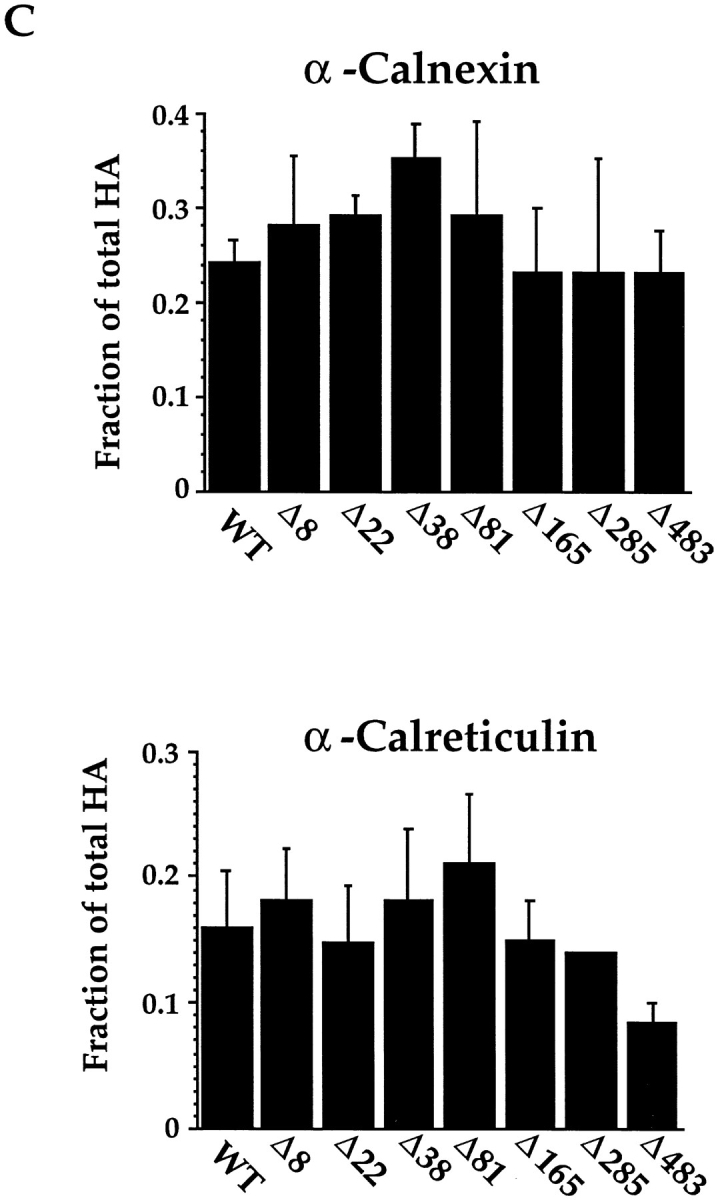
Folding, oligomerization, and calnexin/calreticulin binding of single glycan deletion mutants. (A) Wild-type and single glycosylation mutants of HA were translated under oxidizing conditions in the presence of canine pancreas microsomes at 32°C for 1 h. At the indicated times, samples were removed, alkylated, lysed, and immunoprecipitated with anti-HA antibodies. HA was resolved by nonreducing SDS-PAGE and autoradiography. (B) Translations were performed as above with oxidation carried out for 8 h at 32°C. The alkylated HA was immunoprecipitated with anti-trimer specific HA antibodies and resolved by reducing SDS-PAGE and autoradiography. (C) Translation of 35S-labeled HA were performed as in A for 1 h at 32°C. After alkylation, HA was immunoprecipitated with anti-HA, -calnexin, and -calreticulin antibodies. HA was resolved upon nonreducing and reducing SDS-PAGE and autoradiography. The fraction HA coprecipitating with calnexin or calreticulin was calculated as the amount of anti-calnexin or -calreticulin precipitable HA divided by the total HA precipitated with anti-HA antibodies as described in Fig. 3.