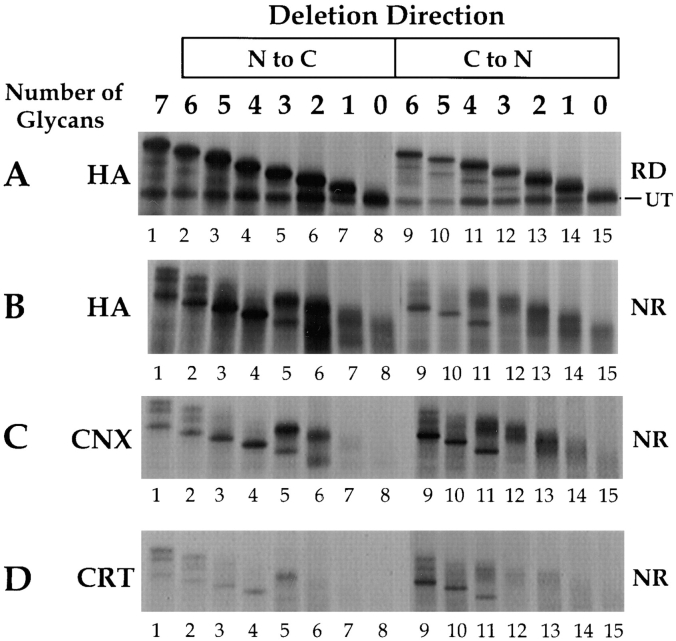
Figure 6.
Determination of the number of glycans required for calnexin or calreticulin binding. Consensus glycosylation sequences were deleted by increasing, in single increments, from the NH2 to the COOH terminus (lanes 2–8), and from the COOH to the NH2 terminus (lanes 9–15). 35S-HA was translated in vitro for 1 h at 32°C, alkylated, lysed, and precipitated with anti-HA, -calnexin (CNX), or -calreticulin (CRT) antisera. The labeled HA was resolved by nonreducing (A, NR) and reducing (B–D, RD) SDS-PAGE and autoradiography. The band designated as UT corresponds to the untranslocated and unglycosylated HA.