Figure 5.
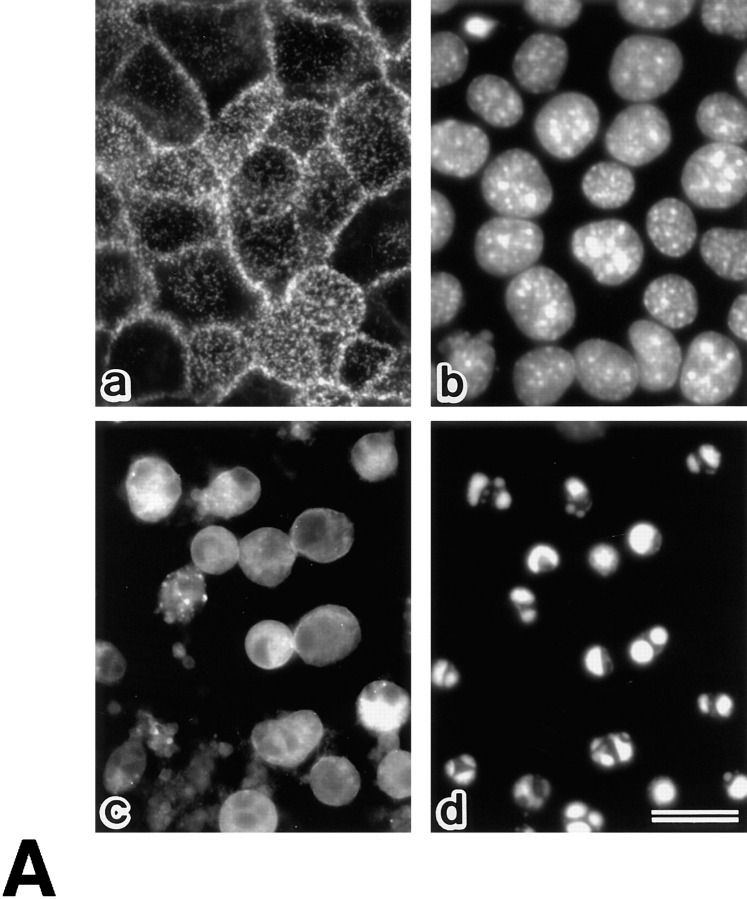
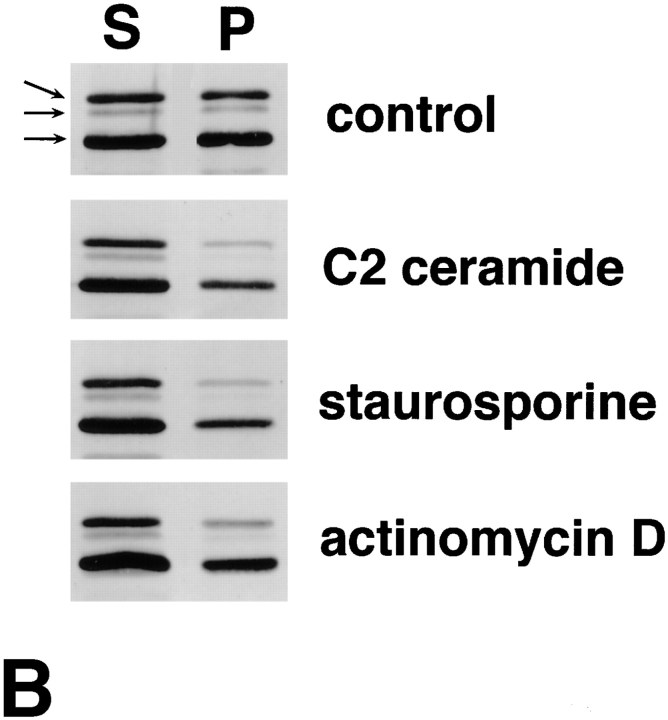
Behavior of ERM proteins during apoptosis in mouse epithelial cells (MTD-1A) and human promyelocytic leukemic cells (HL-60). (A) Immunofluorescence micrographs of MTD-1A cells at 0 h (a and b) and 16 h (c and d) incubation with staurosporine. MTD-1A cells were doubly stained with antiradixin mAb R21 (a and c) and DAPI (b and d). After 16 h of incubation, radixin translocated from apical microvilli and lateral cell–cell contact sites to the cytoplasm, and nuclear changes such as chromatin condensation were observed. Ezrin and moesin showed the same behavior as radixin (data not shown). (B) Solubility of ERM proteins in HL-60 cells at 0 (control) and 16 h incubation with C2 ceramide, staurosporine, or actinomycin D. HL-60 cells were homogenized in physiological saline and centrifuged to separate the soluble and insoluble fractions into the supernatant (S) and pellet (P), respectively. Equivalent amounts of supernatant and pellet were applied to SDS-PAGE and subsequently subjected to immunoblotting with pAb TK89 capable of recognizing all ERM proteins. ERM proteins translocated from the insoluble (P) to the soluble fraction (S) as apoptosis proceeded. Arrows indicate ezrin, radixin, and moesin, respectively, from the top. Bar, 20 μm.