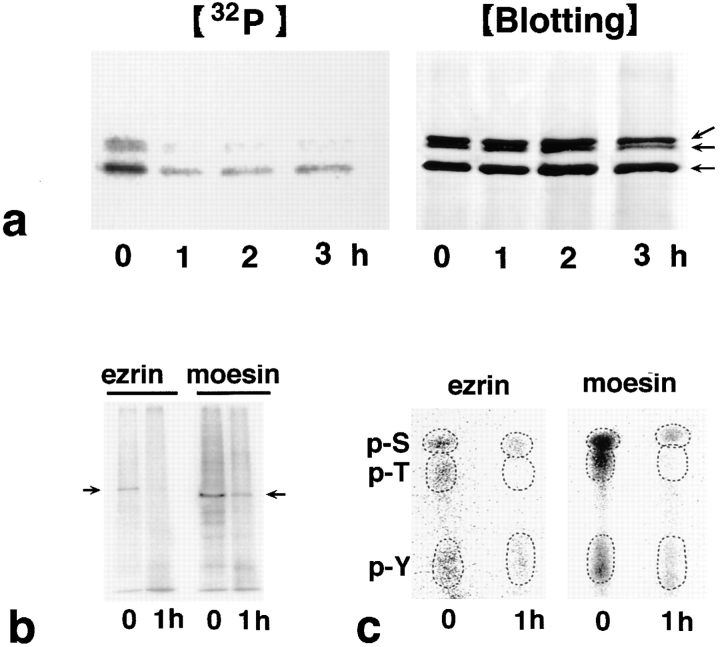
Figure 6.
Dephosphorylation of ERM proteins in LHF cells during Fas-mediated apoptosis. (a) Time course of apoptosis-associated dephosphorylation of ERM proteins. LHF cells were metabolically labeled with [32P]orthophosphate. At 0, 1, 2, and 3 h after FasL stimulation, the cell lysate was immunoprecipitated with anti-ERM pAb, TK89, and analyzed by autoradiography ([32P]) and immunoblotting ([Blotting]). Equal amounts of protein were applied to each lane as revealed in accompanying immunoblots with anti-ERM pAb, TK89. The phosphorylation level of ERM proteins markedly decreased after a 1-h incubation. Arrows indicate ezrin, radixin, and moesin, respectively, from the top. (b) Autoradiography of antiezrin pAb (TK90) or antimoesin mAb (M22) immunoprecipitates from 32P-labeled LHF cells. Immunoprecipitated ezrin and moesin were phosphorylated and dephosphorylated before and 1 h after FasL stimulation, respectively (arrows). (c) Phosphoamino acid analysis. 32P-labeled phosphorylated ezrin or moesin bands (arrows in b) were excised from membranes and processed for phosphoamino acid analysis. The positions of phosphoserine (p-S), phosphothreonine (p-T), and phosphotyrosine (p-Y) were determined by autoradiography through comparison with the ninhydrin staining profiles on unlabeled phosphoamino acid standards. In both ezrin and moesin, phosphothreonine appeared to be preferentially dephosphorylated after 1 h of incubation with FasL, although the levels of phosphoserine and phosphotyrosine were also decreased.