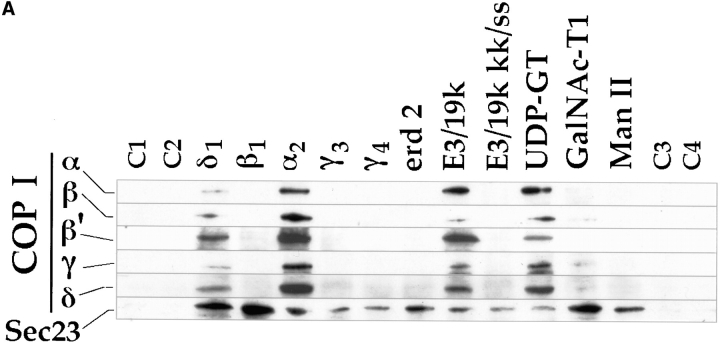
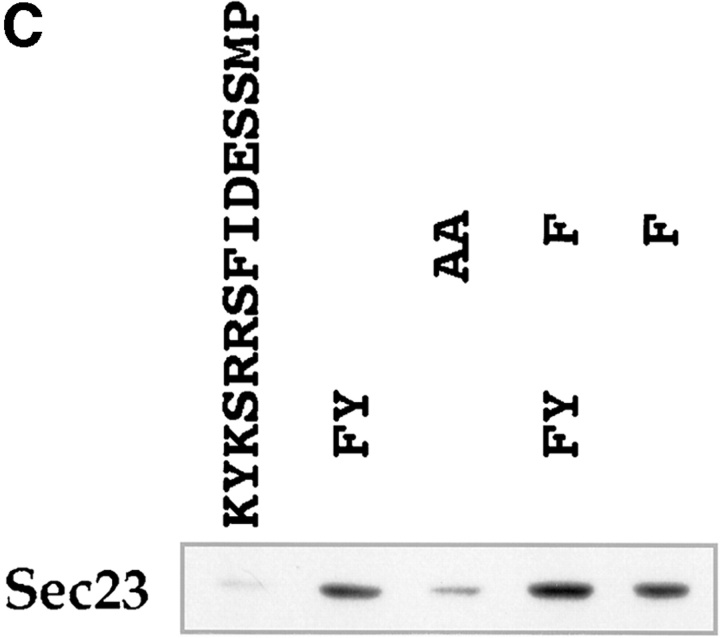
Figure 8.
Binding of COP I and II proteins to cytoplasmic domains. Peptides corresponding to different cytoplasmic domains were coupled to thiopropyl Sepharose beads as described in Materials and Methods, and then assayed for their ability to bind COP I and II. (A) Binding of coat proteins to the cytoplasmic domains of α2, β1, δ1, γ3, γ4, erd2, E3/19k, E3/19k kk/ss (KK mutated to SS), UDP-GT, GalNac-T1, Man II, and control peptides C1-4. Binding of COP I components (tested for were α, β, β′, γ, and δ-COP) to α2 and δ1 was comparable to E3/19k and UDP-GT. The human homologue of Sec23 (hSec23) of the COP II coatomer showed specific binding to all cytoplasmic tails, but not to the four control peptides C1-4. Note the additional binding of the Sec23 to β1 and δ1 as well as to GalNac-TI. (B) Binding of coat proteins as in A but using the cytoplasmic domain of δ1 where pair-wise amino acid substitutions reveal a decrease of COP I binding upon KK-to-SS substitution, whereas Sec23 of COP II is reduced upon FF-to-AA substitution. (C) Introduction of aromatic residues into the mutated E3/19k peptide increases binding of Sec23.