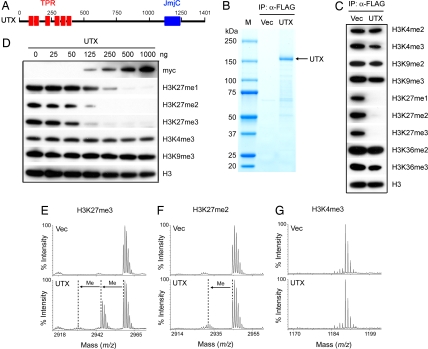
Fig. 1.
UTX specifically demethylates histone H3K27 in vitro. (A) Schematic representation of human UTX protein with six TPR (tetratricopeptide repeat) domains and one JmjC domain. (B) Coomassie blue staining of purified UTX protein. 293T cells were transfected with plasmids expressing FLAG- and myc-tagged UTX or vector only (Vec). Whole-cell extracts were prepared with 1% Nonidet P-40-containing buffer and were incubated with anti-FLAG M2–agarose. After extensive washing, bound proteins were eluted with FLAG peptide, resolved on SDS/PAGE 4–15% followed by Coomassie blue staining. IP, immunoprecipitation. M, protein marker. (C) Eluate corresponding to ≈1 μg of purified UTX was incubated with 2.5 μg of calf core histone in histone demethylase assay. Assay products were analyzed by Western blotting, with antibodies indicated on the right. Where indicated, me1 is monomethyl, me2 is dimethyl, and me3 is trimethyl. (D) Increasing amounts of purified UTX were subjected to histone demethylase assay. (E–G) Mass spectrometry analysis of UTX activity toward H3K27me3 (E), H3K27me2 (F), and H3K4me3 (G) peptides. Three micrograms of UTX was incubated with 1 μg of peptide in histone demethylase assay followed by MALDI-TOF mass spectrometry analysis. The masses of the H3K27me3, H3K27me2, and H3K4me3 peptides were 2,958.7, 2,944.7, and 1,189.7 Da, respectively.