Abstract
Over the course of pregnancy, the human uterus undergoes a 500- to 1,000-fold increase in volume and a 24-fold increase in weight. The uterine smooth muscle layer or myometrium is remodeled, and both cell hypertrophy and hyperplasia are evident. The origin of the new smooth muscle cells, however, is unclear. They may arise from existing smooth muscle cells, or they may be the product of stem cell differentiation. This study describes a subset of myometrial cells isolated from nonpregnant human myometrium that represents the myometrial stem cell population. This was characterized as side population of myometrial cells (myoSP) by a distinct Hoechst dye efflux pattern. In contrast to the main population of myometrial cells (myoMP), myoSP resided in quiescence, underexpressed or lacked myometrial cell markers, and could proliferate and eventually differentiate into mature myometrial cells in vitro only under low oxygen concentration. Although myoMP displayed mature myometrial phenotypes before and after in vitro cultivation, only myoSP, not myoMP, generated functional human myometrial tissues efficiently when transplanted into the uteri of severely immunodeficient mice. Finally, myoSP were multipotent and made to differentiate into osteocytes and adipocytes in vitro under the appropriate differentiation-inducing conditions. Thus, myoSP exhibited phenotypic and functional characteristics of myometrial stem cells. Study of myoSP will improve the understanding of myometrial physiology and the pathogenesis of myometrium-derived diseases such as leiomyoma. myoSP may also represent a novel source of biological material that could be used in the reconstruction of not only the human uterus but also other organs as well.
Keywords: pregnancy, uterus, hypoxia, oxytocin receptor, ATP-binding cassette transporter
The human uterus, which is composed mainly of myometrial cells, exhibits a 20-fold expansion in size over the course of pregnancy. Both myometrial hyperplasia (an increase in cell number) and hypertrophy (an increase in cell size) contribute to the dramatic growth of the pregnant uterus (1, 2). In humans, most growth results from stretch-induced myometrial hypertrophy. Uterine growth during the first weeks of pregnancy, however, is accomplished by myometrial hyperplasia with a smaller contribution from hypertrophy (1). Similarly in rats, myometrial hyperplasia is high during early gestation and decreases dramatically later; and myometrial hypertrophy is low at the beginning of pregnancy but increases as gestation progresses (2). These changes repeat with each successive pregnancy. The presence of stem cells in other areas of the body that undergo continual renewal such as the bone marrow, gut, and skeletal muscle (3) suggests that the changes in the uterus may not be attributable to the hypertrophy and hyperplasia of existing myometrial cells alone. We hypothesize that the myometrium harbors a population of stem cells that enable the repeatable enlargement of the pregnant uterus. In support of this hypothesis we isolated putative myometrial stem cells from the human uterus and demonstrated that they possessed stem cell-like properties including an undifferentiated and quiescent phenotype, the potential for multidifferentiation, and the ability to reconstruct the myometrium in vivo.
Results
We began this study by identifying possible candidates for the myometrial stem cell based on the side-population (SP) phenotype characteristic of the unique ability to efflux the DNA-binding dye Hoechst 33342 via the ATP-binding cassette transporter G2 (ABCG2) (4, 5). SP cells have been isolated from various adult tissues (6–9), demonstrating that this phenotype may represent a common feature of adult stem cells (5). We isolated myoSP from 63 human uterine myometrial specimens and found that these cells represented 2.97 ± 1.13% of the total living cell population (Fig. 1A). Separation of the SP cells was blocked by the addition of 50 μM reserpine, an ABCG2 blocker (Fig. 1A). There was no significant association between the percentage of isolated myoSP and any of the patient parameters including age, parity, contraception, and the day of menstrual cycle when the sample was collected.
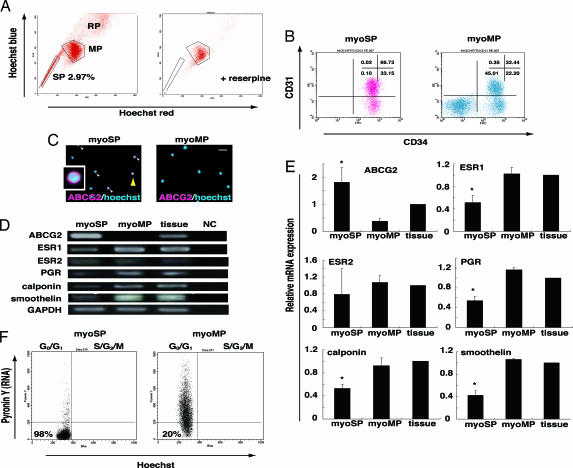
Fig. 1.
Isolation and characterization of human myoSP. (A Left) Distribution of the SP, MP, and replication (RP) populations of Hoechst 33342-stained living cells isolated from human myometrium. (Right) Coaddition of 50 μM reserpine resulted in the disappearance of the myoSP fraction. (B) CD31 and CD34 expression in myoSP and myoMP. (C) ABCG2 expression in myoSP and myoMP as determined by immunocytochemistry. Arrowheads indicate ABCG2-positive cells, one of which, indicated by a yellow arrowhead, is magnified (Inset). (Scale bar, 10 μm.) (D) mRNA expression of ABCG2, ovarian steroid receptors and smooth muscle cell markers in myoSP, myoMP, and whole myometrial tissues as determined by RT-PCR. NC, negative control (no RNA samples). (E) Relative mRNA expression of the ovarian steroid receptors and smooth cell markers in myoSP, myoMP, and whole myometrial tissues was examined by RT-PCR and normalized for GAPDH expression. Each bar indicates the mean + SEM of the relative expression obtained from three independent experiments using three individual samples. *, P < 0.05, versus myoMP. (F) Cell cycle status of myoSP and myoMP was determined by Hoechst 33342 and Pyronin Y staining. The left lower quadrant corresponds to the G0 phase.
We next characterized the myoSP in comparison with the myoMP by analyzing cell surface markers, the expression levels of human myometrium-associated genes, and cell cycle status. Flow cytometry analysis revealed that ≈99% of myoSP, but only 55% of myoMP, were positive for CD34. The CD34+ myoSP was further divided into CD31+ and CD31− cells (67% and 33%, respectively) (Fig. 1B). Although CD34 is a well known stem cell marker for hematopoietic and endothelial cells in human, only 0.11 ± 0.05% (mean ± SD) of the myoSP population were positive for a hematopoietic lineage marker CD45 (data not shown), indicating that the myoSP was not hematopoietic stem cells. Furthermore, myoSP was negative for differentiated endothelial cell markers including CD106, vascular endothelial growth factor-receptor 1, and factor VIII-related antigen [supporting information (SI) Fig. 6A].
In consistent with the flow cytometry data (Fig. 1A), myoSP preferentially expressed ABCG2 protein, as determined by immunofluorescence staining (Fig. 1C). RT-PCR analysis of mRNA derived from isolated myoSP and myoMP demonstrated that the expression level of ABCG2 mRNA was significantly higher in the myoSP than in the myoMP (Fig. 1 C and E). Estrogen receptor-α (ESR1), progesterone receptor (PGR), and the smooth muscle cell markers including calponin and smoothelin were present at very low levels in myoSP compared with both myoMP and whole myometrial tissues (Fig. 1 C and E). This suggests that myoSP represents an immature or undifferentiated population. Finally, estrogen receptor-β (ESR2) was almost undetectable in the myoSP and myoMP fractions and in whole myometrial tissues (Fig. 1D), consistent with ESR2 being relatively absent in the nonpregnant human myometrium (10).
An important characteristic of hematopoietic and other tissue-specific stem cells is that they remain dormant or quiescent, being arrested in the G0 phase of the cell cycle, where they are protected from depletion or exhaustion (11–13). Entry into G1, followed by exit from G0 is associated with an increase in transcription, which can be measured by pyronine Y (PY), an RNA-specific dye. Flow cytometry analysis, followed by costaining with PY and Hoechst 33342 revealed that ≈98% of myoSP but only 20% of myoMP were in the G0 phase (Fig. 1F), which might result from quiescent nature of tissue stem cells.
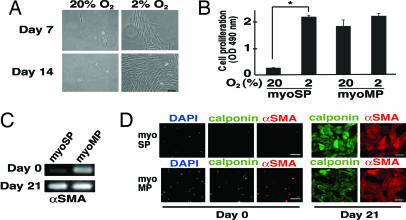
To further characterize myoSP, we cultivated and expanded them in vitro using a variety of culture conditions together with various extracellular matrices including collagen and Matrigel. Because hypoxia is known to exert growth-promoting effects toward some types of stem cells (14, 15), we examined the effects of oxygen concentration upon the proliferation of myoSP and myoMP. Interestingly, although myoSP never proliferated efficiently in vitro in a normoxic (20% O2) environment (Fig. 2A Left), it did proliferate efficiently in vitro under 2% oxygen tension (Fig. 2A Right). Quantitative analysis of cell proliferation revealed that the in vitro expansion of the myoSP fraction under an oxygen tension of 2% was comparable to that of the myoMP (Fig. 2B). Although myoSP did not express smooth cell markers including αSMA and calponin just after isolation, the expression of these markers was induced after 21 days of culture under hypoxic condition (Fig. 2 C and D), suggesting the potential of myoSP for spontaneous differentiation into mature myometrial cells.
Fig. 2.
Hypoxic culture and smooth muscle cell differentiation of myoSP. (A) Phase-contrast images of myoSP cultured for 7 and 14 days under normoxic (20% O2) and hypoxic (2% O2) conditions. (Scale bar, 500 μm.) (B) Effects of hypoxia and normoxia on the proliferation of myoSP and myoMP as determined by the MTS assay. Each bar indicates the mean + SEM of the absorbance at 490 nm obtained from three independent experiments using three individual samples. *, P < 0.05. (C and D) Expression of αSMA mRNA (C) and smooth cell marker proteins (D) in myoSP and myoMP before (day 0) and after 21 days of hypoxic culture (day 21), as determined by RT-PCR (C) and immunofluorescence (D), respectively. (Scale bars, 50 μm.)
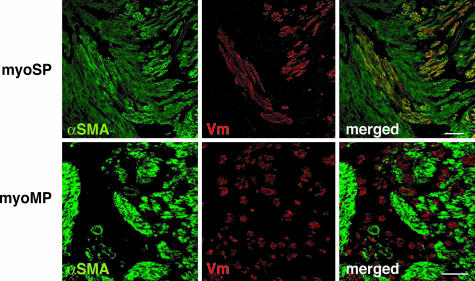
We therefore determined whether myoSP had the potential to reconstitute myometrial tissues in vivo. To address this, myoSP was transplanted into the uteri of NOD/ SCID/γc null (NOG) mice. NOG mice exhibit multiple immunological deficiencies including a lack of cytokine production and functional incompetence of T, B, and NK cells (16). This permits high xenograft engraftment levels as described elsewhere (16, 17). Sixteen NOG mice were ovariectomized and s.c. implanted with an estradiol (E2) pellet. myoSP was then injected into the uterine horn (1 × 105 cells per horn). The same number of age-matched NOG mice was similarly transplanted with myoMP. The uteri were excised 10 weeks after xenotransplantation and subjected to immunofluorescence staining and confocal microscopy. As shown in Fig. 3, human vimentin (Vm)-positive cells were present in all of the uteri of NOG mice transplanted with myoSP. Because a Vm antibody used in this study specifically reacts with human Vm (17) and human nuclear antigen (HNA) were coexpressed with Vm (SI Fig. 7), Vm-positive cells were bona fide of human origin. Importantly, those Vm-positive cells expressing α-smooth muscle cell actin (αSMA), i.e., mature human myometrial cells, were found in 10 of the 16 uteri transplanted with myoSP (Fig. 3 Upper). In contrast, Vm-positive cells were not detected in 13 uteri transplanted with myoMP (data not shown). Only three myoMP-transplanted uteri possessed a small but certain number of Vm-positive cells which, however, never expressed αSMA (Fig. 3 Lower).
Fig. 3.
In vivo reconstitution of myometrium from myoSP in the E2-treated uteri of NOG mice. NOG mice were ovariectomized and xenotransplanted with myoSP or myoMP into their uteri, s.c. implanted with an E2 pellet, and hysterectomized 10 weeks after transplantation. The excised uteri were subjected to immunofluorescence staining using antibodies against αSMA or Vm, followed by DAPI staining. Note that mature myometrial cells expressing αSMA were found in 10 of the 16 uteri transplanted with myoSP but in none of the 16 myoMP-transplanted uteri. Even Vm-positive cells were not detected in 13 uteri transplanted with myoMP (data not shown). Only three myoMP-transplanted uteri possessed a small but certain number of Vm-positive but αSMA-negative cells (Lower). [Scale bars, 50 μm (Upper) and 100 μm (Lower).]
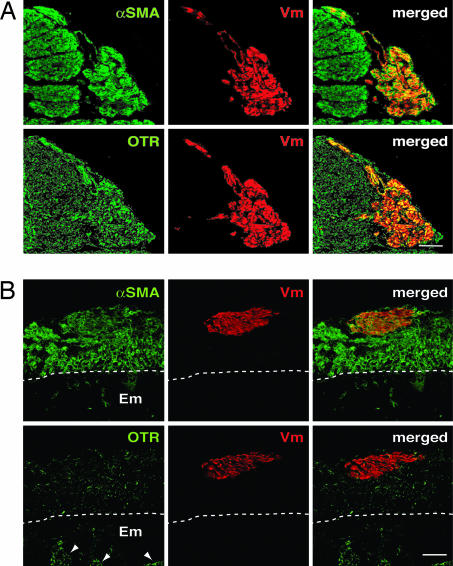
To explore the possible contribution of myoSP to the remodeling and expansion of the uterus during pregnancy, we examined whether myoSP generated tissues resembling the pregnant myometrium in the uteri of the pregnant NOG mice. Up-regulation of oxytocin receptors (OTR) is one of the events associated with “activated” myometrium during late pregnancy and labor in humans and mice (18, 19). The level of OTR mRNA is also gradually increased during the early and mid stages of pregnancy in mice and human (18, 20). Indeed, OTR mRNA can be detected at 7.5 days after conception (d.p.c.) in mice (18). We therefore performed immunofluorescence staining using antibodies against human Vm, αSMA, and OTR on the uteri excised from the pregnant NOG mice at 7.5 d. p.c. who had been xenotransplanted with myoSP or myoMP 2 weeks before mating with Crlj:CD1 (ICR) males.
We found that human-derived cells (Vm-positive) expressing αSMA were present only in the uteri of the NOG mice transplanted with myoSP (Fig. 4A Upper), but not myoMP (data not shown), which was similar to the results of the nonpregnant E2-treated uteri (Fig. 3). Immunofluorescence staining and confocal microscopic analysis of the serial cryosections revealed that the human myometrial cells doubly positive for αSMA and Vm in the pregnant uteri contained a larger number of OTR-positive cells (Fig. 4A Lower and SI Fig. 8) than those present in the nonpregnant E2-treated uteri (Fig. 4B). In agreement with a previous report (21), endometrial glands were positive for OTR (Fig. 4B). Thus, undifferentiated myoSP had not only the potentials of proliferating and differentiating into the mature myometrial cells in the mouse uteri but also the capacity of inducing the expression of OTR particularly in the pregnant uteri.
Fig. 4.
In vivo reconstitution of oxytocin receptor (OTR)-positive myometrium from myoSP in the pregnant uterus of NOG mice. (A) NOG mice were mated to ICR males 2 weeks after transplantation of myoSP into the uterine horn of each mouse. The pregnant uteri were excised at 7.5 d.p.c. and subjected to immunofluorescence staining with antibodies against αSMA, Vm, or OTR, followed by confocal microscopic analysis. (Scale bar, 100 μm.) (B) NOG mice were ovariectomized and xenotransplanted with myoSP into their uteri, s.c. implanted with an E2 pellet, and hysterectomized 10 weeks after transplantation. The excised uteri were analyzed as described in A. Arrowheads and dotted lines indicate endometrial glands and endometrium (Em)–myometrium junctions, respectively. (Scale bar, 100 μm.)
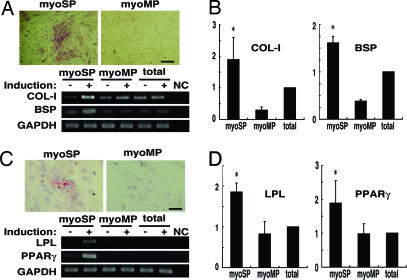
Lastly, we examined the potential of myoSP for multilineage differentiation. We cultured and expanded myoSP and myoMP under hypoxic condition for 2–4 weeks and then replaced the media with “osteogenesis-inducing medium” for induction of osteogenesis or “adipogenic induction and maintenance medium” for adipogenesis, and further cultured under normoxic condition for another 2 weeks. In the presence of osteogenesis-inducing medium, myoSP, but neither myoMP nor unfractionated myometrial cells, exhibited an apparent alkaline phosphatase activity together with significant up-regulation of collagen type 1 (COL-I), bone sialoprotein (BSP), and several other osteogenesis marker genes (Figs. 5 A and B and SI Fig. 9). Similarly, treatment with adipogenic induction and maintenance medium for 2 weeks induced adipogenesis in myoSP, but neither myoMP nor unseparated myometrial cells, as judged by enlarged and rounded morphology, staining with a lipid dye Oil red O, and significant up-regulation of lipoprotein lipase (LPL) and peroxisome-proliferating activated receptor γ (PPARγ) genes, both adipose-specific markers (Figs. 5 C and D).
Fig. 5.
Multidifferentiation capacity of myoSP. (A). Induction of osteocyte differentiation of myoSP, but not myoMP, as determined by alkaline phosphatase staining (Upper) and by RT-PCR for the expression of osteoblast lineage-specific genes (Lower). (Scale bar, 500 μm.) (B) Relative mRNA expression of the indicated osteocyte markers in myoSP, myoMP, and whole myometrial tissues was examined by semiquantitative RT-PCR and normalized for GAPDH expression. Each bar indicates the mean + SEM of the relative expression ratio obtained from three independent experiments using three individual samples. *, P < 0.05, versus myoMP. (C) Induction of adipocyte differentiation of myoSP, but not myoMP, as determined by Oil red-O staining (Upper) and by RT-PCR for the expression of adipocyte lineage-specific genes (Lower). (Scale bar, 250 μm.) (D) Each bar indicates the mean + SEM of the relative mRNA expression of the adipocyte markers from three independent experiments using three individual samples. *, P < 0.05, versus myoMP. Induction (−) and (+) indicate the 21-day treatment with control media and osteocyte- (C) or adipocyte- (D) inducing media, respectively.
Discussion
The results of this study are a strong argument in support of myometrial stem cells. These cells retain the capability to differentiate into multiple cell types as well as smooth muscle cells in vitro and to give rise to myometrial tissues in vivo. We isolated these stem-like or progenitor cells, the myoSP population, from human myometrium and found that they survived and proliferated in vitro only under hypoxic conditions. There is an enlarging body of evidence supporting that low-oxygen conditions enhance the proliferation of adult stem cells and that proliferation in a hypoxic environment is one characteristic that defines the stem cell (14, 15). Thus, the requirement of a hypoxic environment for the survival and expansion of myoSP further substantiates that they are bona fide tissue-specific stem cells.
The requirement of a hypoxic environment for myoSP culture and spontaneous differentiation into smooth muscle cells may implicate myoSP in the pathogenesis of myometrium-derived neoplasms, notably leiomyomas. Leiomyomas are the most common gynecological tumors in women of reproductive age and are associated with a variety of symptoms including abnormal uterine bleeding, pelvic pain, urinary frequency, impaired fertility, and spontaneous abortion. They are clonal in origin (22), and their development is thought to be induced and promoted by hypoxia (23–25). Low oxygen tension (1–5% O2) dramatically up-regulates secreted frizzled-related protein 1, a modulator of Wnt signaling, which exerts antiapoptotic effects in leiomyoma cells but not myometrial cells (25). Myometrial contractions and vasoconstriction that occur during menstruation render the myometrium hypoxic. It is possible that repeated menstruation-induced hypoxia may select a single cell such as a myoSP to proliferate and acquire cytogenetic abnormalities that would ultimately result in the development of a leiomyoma. Leiomyomas occasionally contain adipogenic components and are referred to as lipoleiomyomas (26) and, very rarely, they become ossified (27), which is consistent with the potential of myoSP for differentiation not only into myocytes but also into adipocytes and osteocytes. In support of our idea, Arango et al. (28) have reported that Müllerian duct mesenchyme-specific disruption of β-catenin results in a progressive turnover of uterine myometrium to adipose tissue, suggesting a possible existence of myometrial stem/progenitor cells that have the potential for differentiating into adipocytes in the absence of β-catenin and eventually giving rise to lipoleiomyomas. Indeed, the same group has recently isolated a myometrial SP from the mouse uterus and provided evidence suggesting that the SP contains putative myometrial stem/progenitor cells derived from the Müllerian duct mesenchyme (29), which supports the enrichment of stem/progenitor cells in myoSP in humans.
Human subendometrial myometrium originates from the Müllerian duct, whereas the outer myometrium has non-Müllerian origin (30); however, they both are derived from the mesenchyme (31). In addition to the origin of the myometrium, the present results demonstrating the quiescent and undifferentiated status of myoSP, its multidifferentiation potential, and self-renewal/reconstitution capability suggest that myoSP may share many characteristics of so-called mesenchymal stem cells (MSCs). MSCs are defined as self-renewable, multipotent progenitor cells with the capacity to differentiate into several distinct mesenchymal lineages (32). However, there exist some phenotypic and functional differences. First, although myoSP expressed several MSC markers including CD90, CD73, CD105, and STRO-1 (SI Fig. 6B), the majority of myoSP was CD34-positive and CD44-negative (Fig. 1B and SI Fig. 6B), whereas MSCs are known to be negative for CD34 and positive for CD44 (33). Second, myoMP also expressed STRO-1 like MSCs; however, it did not possess stem cell-like properties. Third, unlike bone marrow- or peripheral blood-derived MSCs (32), myoSP did not generate skeletal muscle cells when we transplanted them into the intact or chemically injured skeletal muscle of NOG mice (SI Fig. 10). Further studies will be required to elucidate a mechanism by which myoSP behaves in a different way from MSCs in response to the microenvironment and/or niche of various tissues and organs.
We here demonstrated that myoSP, whose ESR1 expression level was relatively low, generated myometrial tissues efficiently under the influence of E2. It is conceivable that mouse myometrial ESR-1-positive cells may produce and secret bioactive substances in response to estrogen, which, in turn, may drive transplanted myoSP to proliferate and differentiate into mature myometrial cells in a paracrine manner. In this regard, there may similar mechanism(s) underlying the contribution of myoSP to the hormone-induced remodeling and expansion of human uterus. In support of this idea, Chan et al. (34) have reported that label-retaining cells, which are thought to correspond to quiescent tissue-specific stem cells, are negative for ESR1 in the endometrial epithelium but ESR1-positive in the stroma, suggesting the capacity of the stromal stem/progenitor cells to respond to estrogen and transmit paracrine signals to epithelial cells for endometrial epithelium regeneration. Besides myometrial cell growth, E2 and ESR1 are also involved in the induction of OTR (35), one of the acquired functional properties of myometrium throughout pregnancy, in particular, during the late pregnancy and labor (18–20). In this study, human OTR was dramatically up-regulated in the pregnant uterus compared with nonpregnant E2-treated uterus. These facts collectively raise a possibility that, in addition to E2, certain cell growth-promoting microenvironmental factors of mouse pregnant uterus may contribute to the induction of human OTR in the regenerated human myometrium, and that there may be some similarities in those factors between mouse and human.
A thorough characterization of myoSP is a prerequisite for understanding the complex mechanisms underlying the morphogenesis and physiological regeneration of the myometrium. Our procedures for isolating and cultivating myoSP have made these studies possible. The elucidation of functions and cellular properties of myoSP will broaden our understanding of pathogenesis of myometrium-derived diseases notably leiomyomas. Additionally, the techniques developed by our laboratory for culturing and differentiating myoSP could be a starting point for using these cells for the regeneration of the uterus or other organs.
Materials and Methods
Detailed protocols can be found in SI Methods.
Preparation of Human Myometrial Cells.
Normal myometrial tissues without any abnormalities including adenomyosis or malignancies were obtained from 63 women (age range 35–54 years) undergoing hysterectomy for benign gynecological diseases. The use of these human specimens was approved by the Keio University Ethics Committee, and all patients provided informed consent. The myometrial tissue was cut up manually into small pieces of <1 mm3 which were then incubated for 4–16 h in Dulbecco's modified Eagle's medium (Sigma–Aldrich) containing 0.2% (wt/vol) collagenase (Wako), 0.05% DNase I (Invitrogen), 1% antibiotic-antimycotic mixture (Invitrogen), 10% FBS and 10 mM Hepes buffer solution (Invitrogen) at 37°C on a shaker. After the shaking, the digested tissue was filtered through a sterile 400-μm polyethylene mesh filter to remove undigested tissues, and again filtered through a 40-μm cell strainer (BD–Falcon). The filtrates were layered over Ficoll-Paque PLUS (Amersham Biosciences) and centrifuged to remove red blood cells. The media/Ficoll interface layer was aspirated, washed, and disaggregated in a 0.05% trypsin-EDTA solution (Sigma–Aldrich) containing 0.05% DNase I by pipetting to obtain single-cell suspensions.
Hoechst 33342 and Pyronin Y (PY) Staining.
The dissociated myometrial cells were resuspended at a concentration of 2 × 106 cells per milliliter in SP solution (calcium- and magnesium-free Hanks' balanced salt solution containing 2% FBS, 1% penicillin/streptomycin, and 10 mM Hepes). Hoechst 33342 (Sigma–Aldrich) was then added at a final concentration of 5 μg/ml and the sample incubated at 37°C for 90 min. A parallel aliquot was stained with Hoechst dye in the presence of 50 μM reserpine (Sigma–Aldrich). After incubation, the cells were centrifuged at 1,500 × g for 7 min, resuspended in 2 ml of cold SP solution and further incubated with 1 μg/ml propidium iodide (PI; Sigma–Aldrich) to label nonviable cells. The cells were kept on ice at all times after staining with the Hoechst 33342 dye. The Hoechst dye- and PI-treated cells were subjected to flow cytometric analysis to separate the myoSP and myoMP. For costaining with Hoechst 33342 and PY, sorted myoSP and myoMP were washed twice in SP solution, incubated with 1 μg/ml Hoechst33342 together with 50 μM reserpine in a 37°C water bath for 45 min, and then, without any additional washing, incubated with 3.3 μM PY (Polysciences) for another 45 min. The cells were washed once in an excess volume of SP solution and subjected to FACS analysis. Cells cotreated with Hoechst and reserpine or treated with PY alone were used as negative controls. PY was excited with an argon laser.
Antibody Staining for FACS Analysis.
Hoechst-stained single myometrial cells were resuspended in SP solution at 1–5 × 107 cells per milliliter. The antibodies used for FACS were conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), or allophycocyanin (APC) (SI Table 1). All incubations with antibodies were carried out on ice for 30 min. After antibody staining, the cells were washed with an excess amount of SP solution and resuspended in SP solution at a concentration of 1 × 107 cells per milliliter before FACS analysis.
FACS Analysis.
Myometrial cells were sorted by a FACS Vantage SE flow cytometer (BD Biosciences) and analyzed with Cell-Quest software (BD Biosciences). After collecting 5 × 104 events, the SP and MP populations were defined as reported previously (4, 9) (for details see SI Methods).
RT-PCR.
The primers used for PCR amplification are as listed in SI Table 2. Total RNA was extracted by using TRIzol Reagent (Invitrogen) and reverse transcribed with SuperScript III reverse transcriptase (Invitrogen) and random hexamers, according to the manufacturers' instructions. cDNA was synthesized from 60,000 to 200,000 myoSP or myoMP. An aliquot was then assayed for the relative amount of GAPDH signal. These data were then used to calculate a dilution factor for each sample, so that each contained the same concentration of GAPDH cDNA.
Cell Culture.
Both myoSP and myoMP were cultured in MSC growth medium (MSCGM; Cambrex Bio Science) under normoxic (20% O2) or hypoxic (2% O2) conditions for 4 weeks. Cell proliferation activities were measured by using the Cell Titer 96 Aqueous One Solution Cell Proliferation Assay (Promega) according to the manufacturer's instructions.
For induction of differentiation, myoSP and myoMP were plated at a density of ≈5 × 103 cells per well in 96-well dishes with MSCGM and grown in a hypoxic environment until the cells reached confluence (14–28 days). Subsequently, the cultures were exported to a normoxic environment, fed with osteocyte differentiation media or adipogenic induction/maintenance media (Cambrex Bio Science) for 2 to 3 weeks, and then harvested for RNA extraction, or subjected to alkaline phosphatase staining or Oil red O staining (for details see SI Methods).
Transplantation Analysis.
All experiments using NOG mice (Central Institute for Experimental Animals) were conducted in accord with the Guide for the Care and Use of Laboratory Animals of the Keio University School of Medicine. At transplantation, both recipient ovaries were removed to eliminate the influence of endogenous estrogen and the animals s.c. implanted with single E2 pellet (1.5 mg E2 per pellet, Innovative Research of America). We have previously reported that the serum E2 level in mice implanted with single E2 pellet was 216 ± 144 pg/ml (mean ± SE, n = 3) (17). myoSP (5 × 104 cells) were injected into each uterine horn of 16 NOG mice by using a 29-gauge needle. The myoMP were similarly transplanted into 16 age-matched NOG mice. The uteri were excised 10 weeks after transplantation. Alternatively, NOG mice were mated to ICR males 2 weeks after myoSP or myoMP (5 × 104 cells) were injected into the uterine horn of each NOG mouse. The pregnant uteri were excised at 7.5 d.p.c.
Immunofluorescence and Confocal Microscopy.
Immunofluorescence analyses were performed on cytospun myoSP and myoMP or cryosections derived from uteri transplanted with myoSP or myoMP. Glass slides onto which the cytospun cells were overlaid or sections were mounted were fixed with 4% PFA for 20 min and washed with PBS, followed by permeablization with 0.2% Triton X-100 in PBS for 10 min. After blocking with 10% FBS for 60 min, slides were successively stained with various antibodies as listed in SI Table 1, followed by incubation with secondary antibodies conjugated with Alexa Fluor 488 (Molecular Probes) or red fluorescent dye Cy3 (Sigma–Aldrich) to visualize the primary antibodies. Nuclei were stained by using Bisbenzimide H33258 (Sigma–Aldrich) or TOTO3 (Molecular Probes). Images were collected by using an inverted Leica DMIRE2 fluorescent microscope (Leica Microsystems) equipped with a CCD camera (VB-700; Keyence) and a Leica TCS SP2 confocal microscopy system. Some acquired images were subjected to three-dimensional reconstruction using LCS software (Leica).
Statistical Analysis.
A P value was calculated by using the unpaired Student t test.
Supplementary Material
Acknowledgments
We thank Shouka Kan and the other gynecologists of Keiyu Hospital (Yokohama, Japan) for collection of human myometrial samples and Norikazu Tamaoki, Takayuki Ohkawa, Sadafumi Suzuki, and the members of T.M.'s, Y.M.'s, and H.O.'s laboratories for their experimental support. This study was supported, in part, by Grants-in-Aid from the Japan Society for the Promotion of Science (to T.M., Y.Y., and M.O.), by a National Grant-in-Aid for the Establishment of High-Tech Research Center in a Private University (to T.M.), and by a Grant-in-Aid from the 21st Century Centers of Excellence program of the Ministry of Education, Science, and Culture of Japan at Keio University.
Abbreviations
- ABCG2
ATP-binding cassette transporter G2
- APC
allophycocyanin
- αSMA
α-smooth muscle actin
- BSP
bone sialoprotein
- COL-I
collagen type 1
- ESR1
estrogen receptor-α
- ESR2
estrogen receptor-β
- HNA
human nuclear antigen
- LPL
lipoprotein lipase
- MSC
mesenchymal stem cell
- myoMP
main population of myometrial cells
- myoSP
side population of myometrial cells
- NOG
NOD/SCID/γc null
- OTR
oxytocin receptor
- PE
phycoerythrin
- PGR
progesterone receptor
- PI
propidium iodide
- PPARγ
peroxisome-proliferating activated receptor γ
- PY
pyronine Y
- SP
side population
- Vm
vimentin.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704472104/DC1.
References
- 1.Ramsey EM. Anatomy of the Human Uterus. Cambridge, UK: Cambridge Univ Press; 1994. pp. 18–40. [Google Scholar]
- 2.Shynlova O, Oldenhof A, Dorogin A, Xu Q, Mu J, Nashman N, Lye SJ. Biol Reprod. 2006;74:839–849. doi: 10.1095/biolreprod.105.048124. [DOI] [PubMed] [Google Scholar]
- 3.Körbling M, Estrov Z. N Engl J Med. 2003;349:570–582. doi: 10.1056/NEJMra022361. [DOI] [PubMed] [Google Scholar]
- 4.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Challen GA, Little MH. Stem Cells. 2006;24:3–12. doi: 10.1634/stemcells.2005-0116. [DOI] [PubMed] [Google Scholar]
- 6.Redvers RP, Li A, Kaur P. Proc Natl Acad Sci USA. 2006;103:13168–13173. doi: 10.1073/pnas.0602579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schienda J, Engleka KA, Jun S, Hansen MS, Epstein JA, Tabin CJ, Kunkel LM, Kardon G. Proc Natl Acad Sci USA. 2006;103:945–950. doi: 10.1073/pnas.0510164103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montanaro F, Liadaki K, Volinski J, Flint A, Kunkel LM. Proc Natl Acad Sci USA. 2003;100:9336–9341. doi: 10.1073/pnas.1133179100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuzaki Y, Kinjo K, Mulligan RC, Okano H. Immunity. 2004;20:87–93. doi: 10.1016/s1074-7613(03)00354-6. [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi H, Fujimoto J, Aoki I, Tamaya T. Steroids. 2003;68:11–19. doi: 10.1016/s0039-128x(02)00111-3. [DOI] [PubMed] [Google Scholar]
- 11.Quesenberry PJ, Colvin GA, Lambert JF. Blood. 2002;100:4266–4271. doi: 10.1182/blood-2002-04-1246. [DOI] [PubMed] [Google Scholar]
- 12.Young HE. Curr Top Microbiol Immunol. 2004;280:71–109. doi: 10.1007/978-3-642-18846-6_2. [DOI] [PubMed] [Google Scholar]
- 13.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Zhu LL, Wu LY, Yew DT, Fan M. Mol Neurobiol. 2005;31:231–242. doi: 10.1385/MN:31:1-3:231. [DOI] [PubMed] [Google Scholar]
- 15.Grayson WL, Zhao F, Izadpanah R, Bunnell B, Ma T. J Cell Physiol. 2006;207:331–339. doi: 10.1002/jcp.20571. [DOI] [PubMed] [Google Scholar]
- 16.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, et al. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 17.Masuda H, Maruyama T, Hiratsu E, Yamane J, Iwanami A, Nagashima T, Ono M, Miyoshi H, Okano HJ, Ito M, et al. Proc Natl Acad Sci USA. 2007;104:1925–1930. doi: 10.1073/pnas.0604310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimura T, Takemura M, Nomura S, Nobunaga T, Kubota Y, Inoue T, Hashimoto K, Kumazawa I, Ito Y, Ohashi K, et al. Endocrinology. 1996;137:780–785. doi: 10.1210/endo.137.2.8593830. [DOI] [PubMed] [Google Scholar]
- 19.Kubota Y, Kimura T, Hashimoto K, Tokugawa y, Nobunaga K, Azuma C, Saji F, Murata Y. Mol Cell Endocrinol. 1966;124:25–32. doi: 10.1016/s0303-7207(96)03923-8. [DOI] [PubMed] [Google Scholar]
- 20.Siebel AL, Gehring HM, Reytomas IG, Parry LJ. Endocrinology. 2003;144:4272–4275. doi: 10.1210/en.2003-0548. [DOI] [PubMed] [Google Scholar]
- 21.Takemura M, Nomura S, Kimura T, Inoue T, Onoue H, Azuma C, Saji F, Kitamura Y, Tanizawa O. Endocrinology. 1993;132:1830–1835. doi: 10.1210/endo.132.4.8384999. [DOI] [PubMed] [Google Scholar]
- 22.Walker CL, Stewart EA. Science. 2005;308:1589–1592. doi: 10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]
- 23.Fujii S, Konishi I, Horiuchi A, Orii A, Nikaido T. Mesenchymal Cell Differentiation: Speculation About the Histogenesis of Uterine Leiomyomas. New York: Parthenon; 1999. pp. 3–15. [Google Scholar]
- 24.Pavlovich CP, Schmidt LS. Nat Rev Cancer. 2004;4:381–393. doi: 10.1038/nrc1364. [DOI] [PubMed] [Google Scholar]
- 25.Fukuhara K, Kariya M, Kita M, Shime H, Kanamori T, Kosaka C, Orii A, Fujita J, Fujii S. J Clin Endocrinol Metab. 2002;87:1729–1736. doi: 10.1210/jcem.87.4.8375. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Kumar D, Seidman JD. Int J Gynecol Pathol. 2006;25:239–242. doi: 10.1097/01.pgp.0000192273.66931.29. [DOI] [PubMed] [Google Scholar]
- 27.Bhattacharya N, Banerjee AK, Sengupta J. J Indian Med Assoc. 1998;96:99. [PubMed] [Google Scholar]
- 28.Arango NA, Szotek PP, Manganaro TF, Oliva E, Donahoe PK, Teixeira J. Dev Biol. 2005;288:276–283. doi: 10.1016/j.ydbio.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 29.Szotek PP, Chang HL, Zhang L, Preffer F, Dombkowski D, Donahoe PK, Teixeira J. Stem Cells. 2007;25:1317–1325. doi: 10.1634/stemcells.2006-0204. [DOI] [PubMed] [Google Scholar]
- 30.Gargett CE. Hum Reprod Update. 2007;13:87–101. doi: 10.1093/humupd/dml045. [DOI] [PubMed] [Google Scholar]
- 31.Konishi I, Fujii S, Okamura H, Mori T. J Anat. 1984;139(Pt 2):239–252. [PMC free article] [PubMed] [Google Scholar]
- 32.He Q, Wan C, Li G. Stem Cells. 2007;25:69–77. doi: 10.1634/stemcells.2006-0335. [DOI] [PubMed] [Google Scholar]
- 33.Deans RJ, Moseley AB. Exp Hematol. 2000;28:875–884. doi: 10.1016/s0301-472x(00)00482-3. [DOI] [PubMed] [Google Scholar]
- 34.Chan RW, Gargett CE. Stem Cells. 2006;24:1529–1538. doi: 10.1634/stemcells.2005-0411. [DOI] [PubMed] [Google Scholar]
- 35.Gimpl G, Fahrenholz F. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.