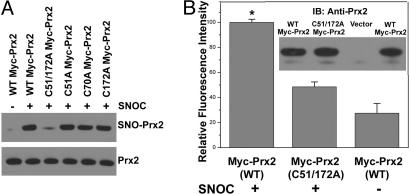
Fig. 1.
S-nitrosylation of Prx2 in vitro and in vivo. (A) (Upper gel) Cell lysates from human SH-SY5Y cells were incubated in various concentrations of SNOC at room temperature, followed by assay for SNO-Prx2. SNO-Prx2 was detected by biotin-switch assay 30 min after SNOC exposure. (Lower gel) Total Prx2 in cell lysates by Western blot analysis. (B) (Upper gel) Human SH-SY5Y cells were exposed to 200 μM SNOC or control for 30 min, followed by assay for SNO-Prx2. Control samples were subjected to decayed (old) SNOC or SNOC but not to ascorbate. (Lower gel) Total Prx2. (C) Stability of SNO-Prx2 in SH-SY5Y cells. Thirty minutes after incubation in 200 μM SNOC, the medium was replaced and cells were cultured for an additional 8 or 24 h. SNO-Prx2 was detected by the biotin-switch assay. (D) (Upper gel) HEK-293 cells stably expressing nNOS were assayed for endogenous SNO-Prx2. nNOS was activated by the addition of 5 μM Ca2+ ionophore A23187 in the presence or absence of NNA. Activation of nNOS increased endogenous SNO-Prx2, and NNA prevented this increase. (Lower gel) Total Prx2. (E) Primary rat cortical neurons were exposed to 200 μM SNOC, and SNO-Prx2 was detected by the biotin-switch assay. Control samples were subjected to decayed (old) SNOC or SNOC but not to ascorbate. (F) SNO-Prx2 was detected in an NOS-dependent manner in primary mouse cortical cultures exposed to NMDA. Lanes 1–4 are samples from WT neurons and lane 5 from nNOS KO neurons.